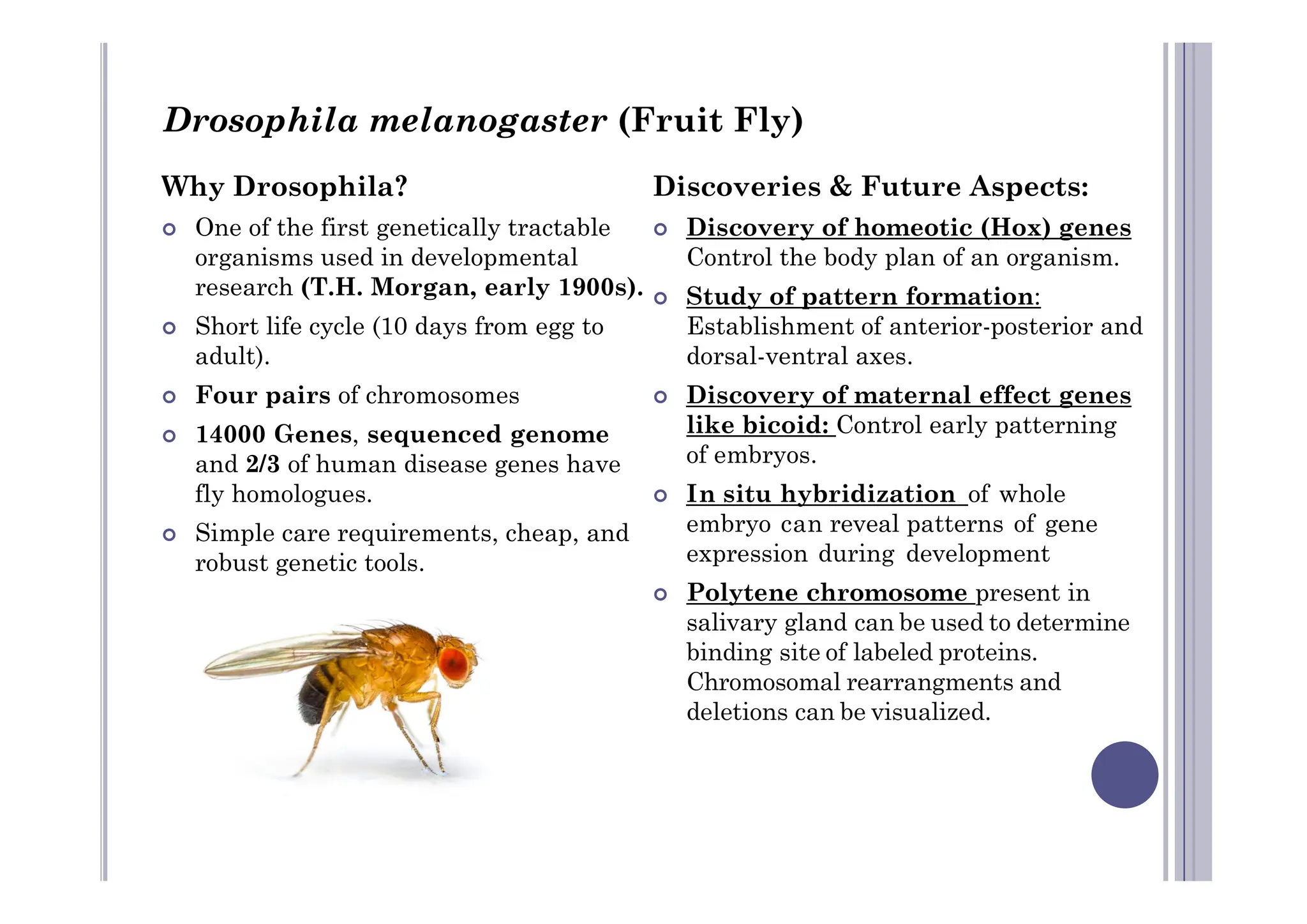
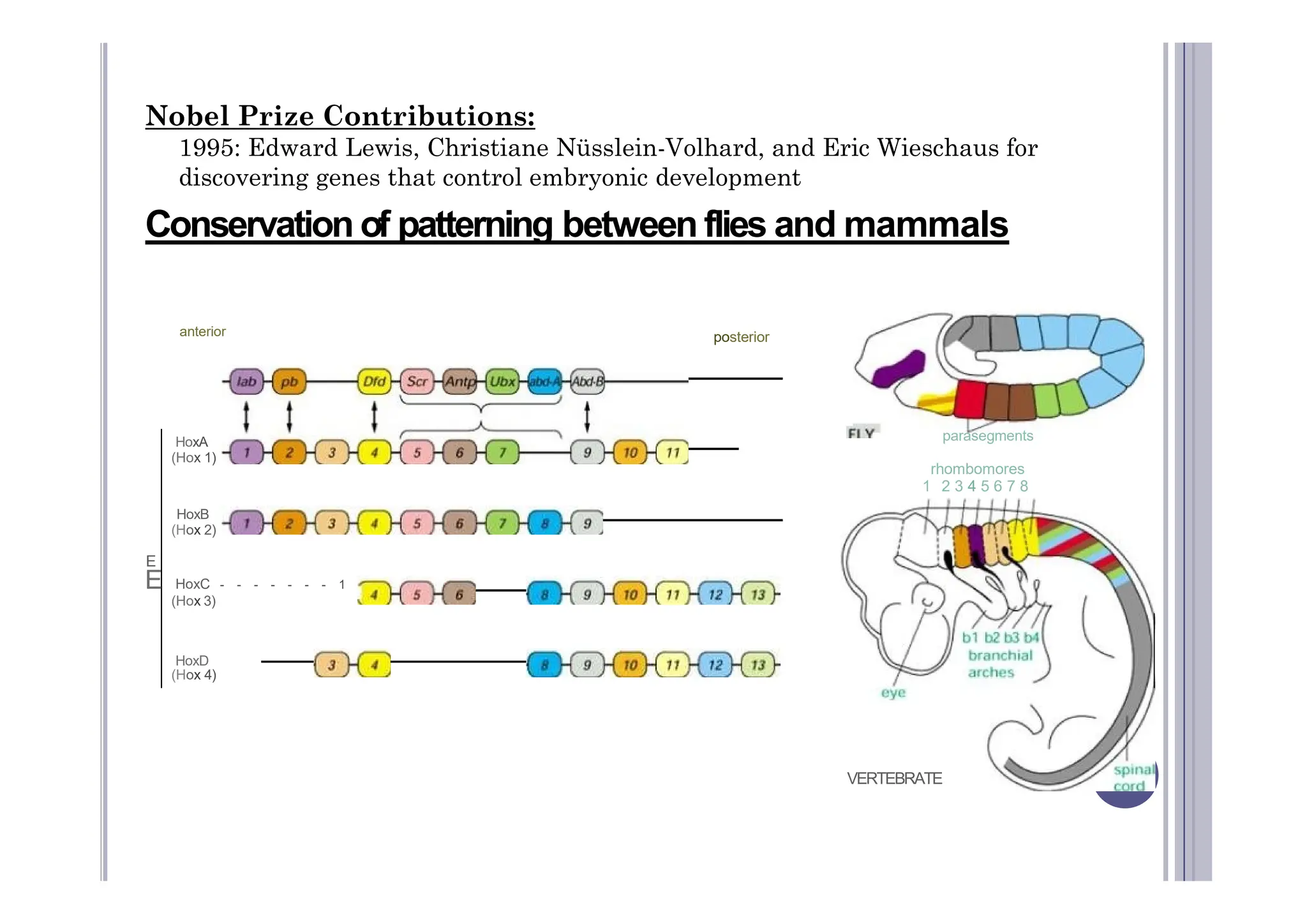
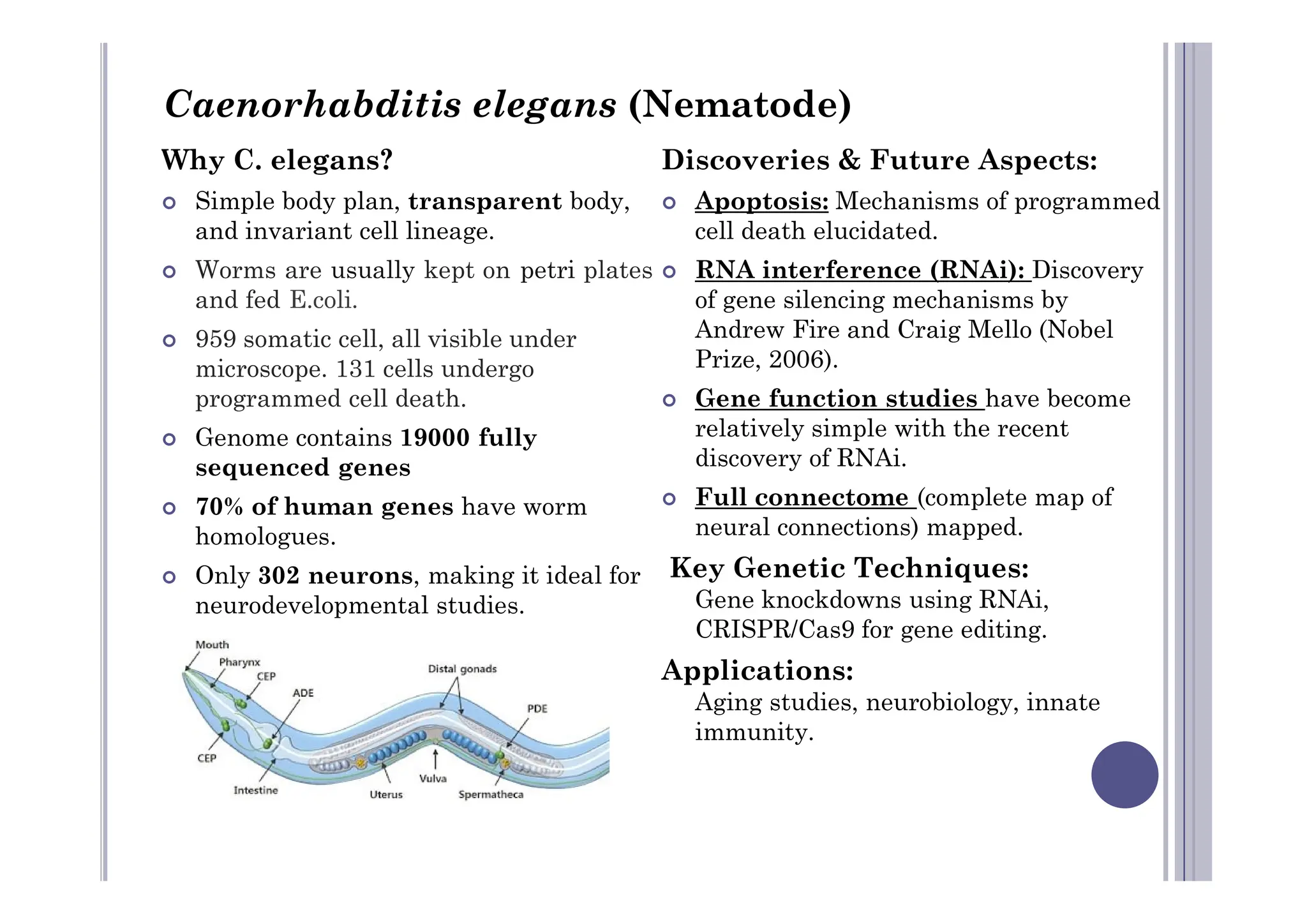
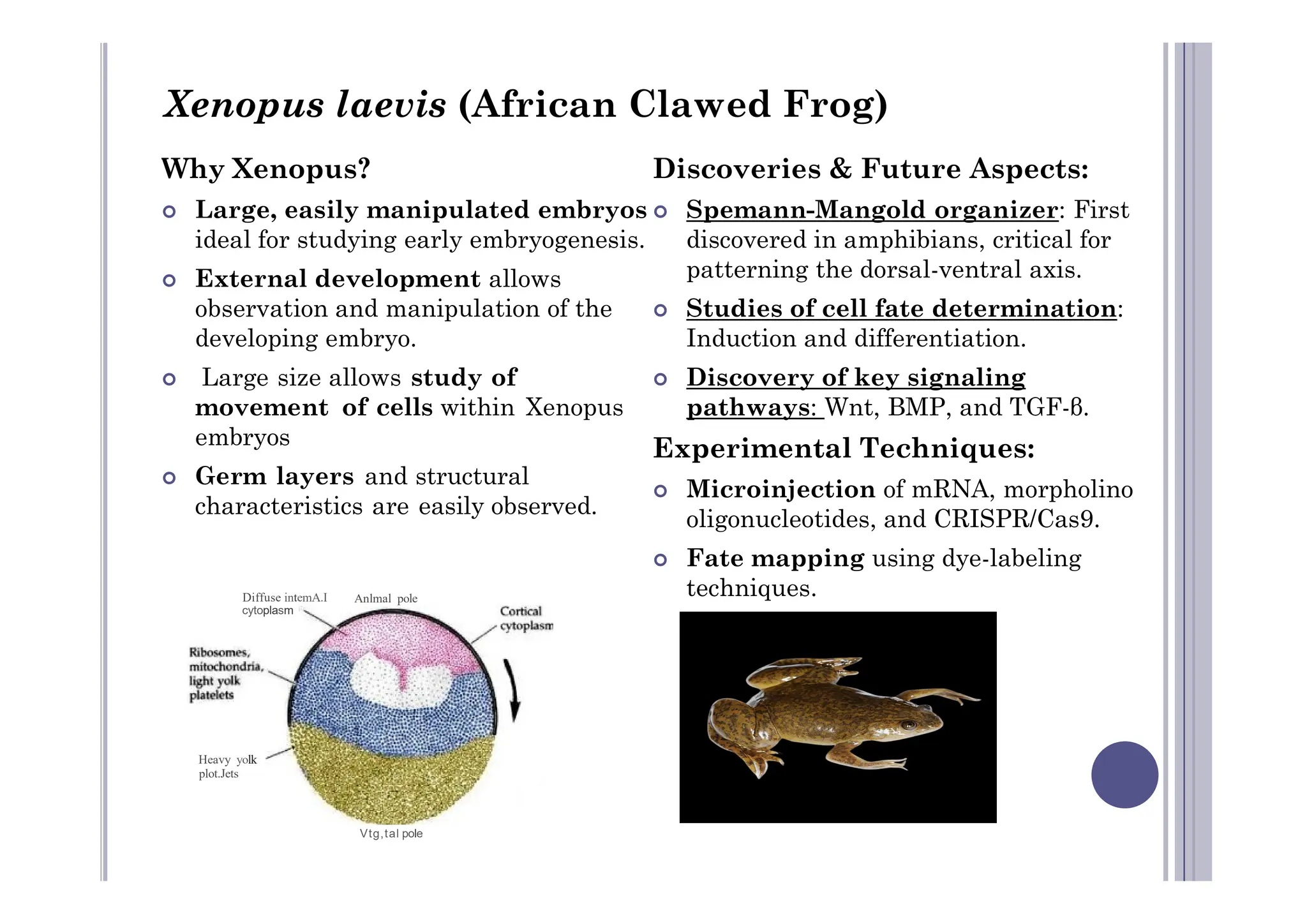
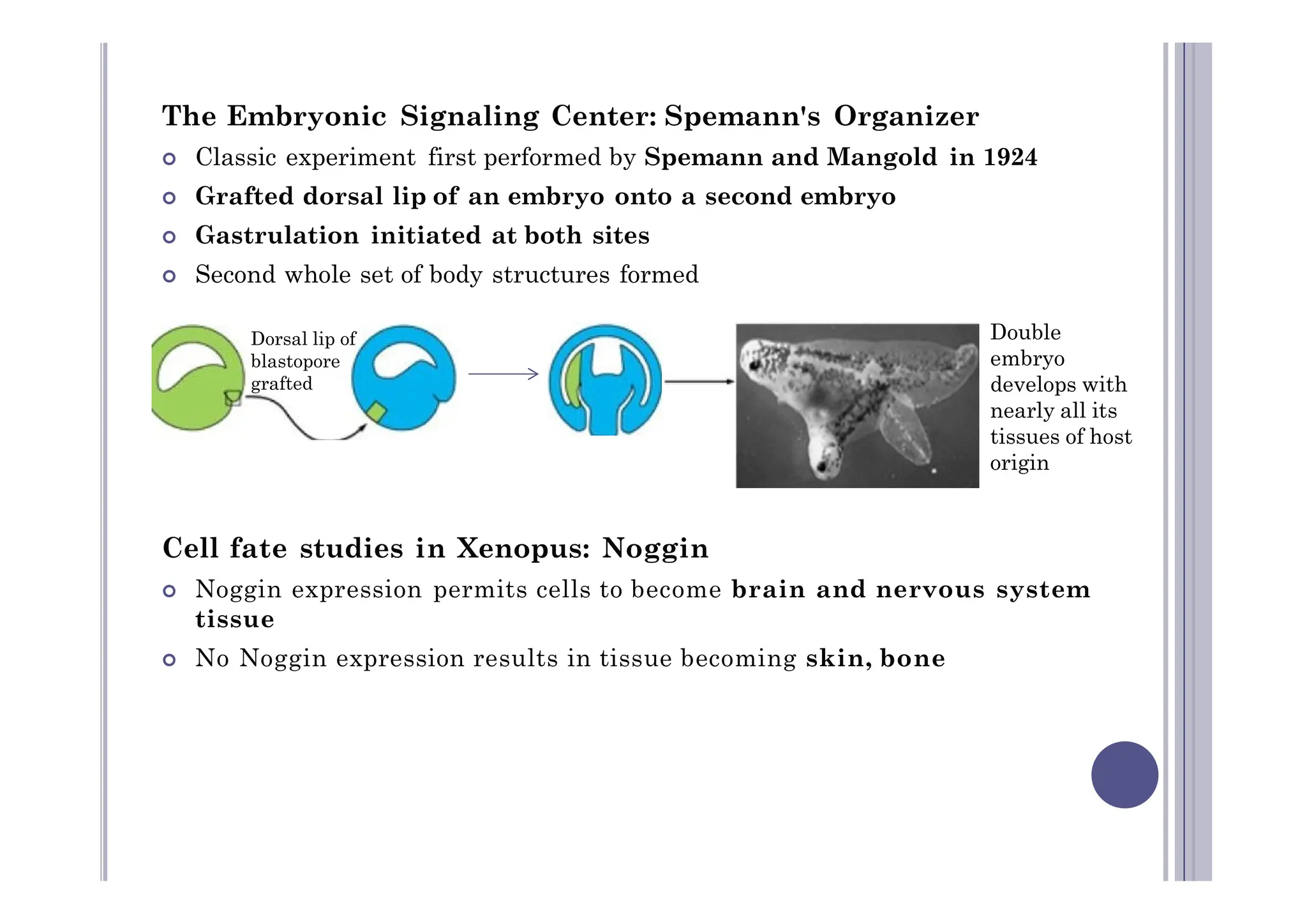
Experimental developmental biology explores how organisms develop through controlled experiments, focusing on cellular differentiation and morphogenesis using model organisms due to their practicality and genetic homology to humans. Model organisms like Drosophila, C. elegans, zebrafish, and mice are pivotal in studying developmental processes and genetic phenomena, yielding vital discoveries in fields like neurobiology and gene regulation. This research is essential for advancing our understanding of human biology and disease through insights gained from the simpler systems of these model organisms.