dec30updateddocument1023_ireallynee.docx
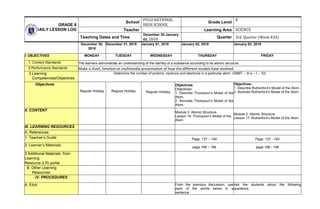
- 1. GRADE 8 DAILY LESSON LOG School PULO NATIONAL HIGH SCHOOL Grade Level 8 Teacher Learning Area SCIENCE Teaching Dates and Time December 30-January 03, 2018 Quarter 3rd Quarter (Week #26) December 30, 2018 December 31, 2018 January 01, 2018 January 02, 2018 January 03, 2018 I. OBJECTIVES MONDAY TUESDAY WEDNESDAY THURSDAY FRIDAY 1. Content Standards The learners demonstrate an understanding of the identity of a substance according to its atomic structure. 2.Performance Standards Make a chart, timeline or multimedia presentation of how the different models have evolved. 3.Learning Competencies/Objectives Determine the number of protons, neutrons and electrons in a particular atom. (S8MT – III e – f – 10) Objectives Regular Holiday Regular Holiday Regular Holiday Objectives: Objectives: 1. Describe Thompson’s Model of the Atom. 2. Simulate Thompson’s Model of the Atom. Objectives: 1. Describe Rutherford’s Model of the Atom. 2. Illustrate Rutherford’s Model of the Atom. II. CONTENT Module 2: Atomic Structure Lesson 16: Thompson’s Model of the Atom Module 2: Atomic Structure Lesson 17: Rutherford’s Model of the Atom III. LEARNING RESOURCES A. References 1. Teacher’s Guide Page. 137 – 140 Page. 137 - 140 2. Learner’s Materials page 198 – 199 page 198 - 199 3.Additional Materials from Learning Resource (LR) portal B. Other Learning Resources IV. PROCEDURES A. Elicit From the previous discussion, use each of the words below in a sentence. Ask the students about the following questions:
- 2. Attract Repel Neutral Who knows how to play billiard? What will you do to scatter the ball? How forceful is it to hit the ball? B .Engage During one of your visits to SM Mall, your family decided to take their snacks at Chowking and ordered a set of siopao. How do you describe the siopao? When the idea of the atom was first propose by the ancient Greeks , how do these scientists intend to discover about the atoms? Let the students illustrate Rutherford’s model of the atom based on the preliminary activity. What do you think are the chances of the alpha particle directly hitting the nucleus? (compare hitting the ball and hitting the nucleus) C. Explore Let the student perform the activity below. “Small but terrible” 1. Get the activity box from your teacher. Write the box number on your worksheet. Inside the box are the “mystery object” which is fixed in place and one marble. Without opening the box, guess the shape, size and location of the mystery object. Title of the Activity:“Unmixed Me” In the class, let the students work in groups. Tell them to do this activity on the area assigned to them. Instruct them to write their output on a piece of manila paper. Follow the procedure below. Procedures: 1. Scatter the 20 pieces marble in a circle on the floor about one foot in diameter. Imagine these to be the electrons in the Thompson’s raisinbread model of the atom. 2. As forcefully as you can, slide the rubber ball to hit the circle of marbles. Imagine the rubber ball to be the high speed alpha particle in Rutherford’s experiment.
- 3. Using the representation of the atoms of the gold foil, draw the path of the positively charged alpha particles as they moved through the atoms. When all the groups are done with their activity, let them present it in front of the class. Make sure that they will answer guide questions based on their output. D. Explain 1. Were you able to determine the size, shape and location of the mystery object? Draw a picture of the mystery box. 2. Were you able to infer the size, shape and location of the mystery object in the box? 3. How close was your guess? If given the chance to guess another mystery object, will you change your strategy? If yes, what changes will this be? 4. What made you decide to group these pictures? 5. What insights did you obtain when you compare this activity with the previous one? 6. Can you say that this activity is the reversed of the previous activity? Why did you say so? Guide Questions: 1. When you slide the rubber ball over the circle of marbles, what did you observe? 2. What happened to the rubber ball as it hits the circle of marbles? 3. If you repeat what you did with the rubber ball and the marbles many times, do you think you will have the same observation with the previous one? Facilitate a brief discussion about their output. E. Elaborate Explain Thomson’s model of atom using this picture Rutherford’s team proposed the Nuclear Model of Atom using his gold foil experiment which established the existence of the nucleus of the atom. He offered the following rationalizations: 1. An atom consists of a large empty space is indicated by the ease with which alpha particles passed through it.
- 4. .J. Thompson discovered that atoms have negatively – charged particles which he called electrons. It led him to propose a new model for the atom which he called the plum pudding model. He proposed that the negatively – charged electrons were embedded in a kind of cloud of positive charge. In the Philippines, the model is known as raisin bread model. 2. An atom consists of a positive region which could have been hit by the alpha particles that deflected. 3. The positive region in an atom that corresponds to a very small but massive portion. The very few alpha particles that bounced back could have directly hit and were repelled by the positive nucleus of the atom F. Evaluate Read the questions carefully. Write the correct answer on a ¼ sheet of paper. 1. Thompson proposed that the atom is a mass of positive charges with the electrons scattered throughout it. What do you call to this model? a. Plum Pudding Model b. Alpha – Particle Scattering Model c. Dalton’s Model of Atom d. Rutherford’s Model 2. Thompson simulated in his Raisin – pudding model that the positive mass is the pudding. How about the raisins? a. electrons c. neutrons b. protons d. nucleus 3. Given the Thompson’s model of an atom, what can you infer from the figure? Multiple Choice Assessment Direction:Read the questions carefully. Write the correct answer on a ¼ sheet of paper. 1. What is in the center of the Rutherford model? a. multiple electrons c. neutrons b. single proto d. nucleus 2. In Rutherford atomic model the alpha particles were stroked on ________. a. aluminum b. gold c. silver d. titanium 3. The particles which were deflected backwards in Rutherford's experiments were hit upon by ______. a. nucleusc. electrons b. empty space d. none of the above 4. In Rutherford's experiment, a thin gold foil was bombarded with alpha particles. What happens when a high speed alpha particle directly hits the
- 5. Checked by: MARIA CRISTINA F. LIM Ed.,D. MASTER TEACHER III/SCIENCE a. The atom is a mass of positive charge with electrons scatted throughout it. b. It was the same with Dalton’s model of atom. c. The model also pertains to matter. d. The model has something to do with the chemical combination of elements. 4. Which of the following scientists developed the plum – pudding model of the atom? a. John Dalton b. Robert Milikan c. J.J. Thompson d. Ernest Rutherford 5. Which of these is the conclusion from J. J Thomson's experiment? a. Electrons travel in a straight line. b. Electrons emit light. c. Atoms of some metals do not have electrons. d. Atoms of all metals have electrons. positively charged nucleus? a. Alpha particles are strongly deflected since it hits a particle with bigger mass. b. Alpha particles bounced straight back from thegold foil. c. Alpha particles passed through the foil with little deflection. d. Alpha particles become embedded in the foil. 5. Which of these expectations of Rutherford was based on J.J Thomson's model before conducting his own experiment? a. Alpha particles would break into smaller particles after striking the gold foil. b. Alpha particles would pass through the gold foil with little or no deflection. c. Alpha particles would bounce back along the same path as they travel to the gold foil. d. Alpha particles would embed themselves as they strike the gold foil. G. Extend Create a 3D model of Thomson’s atomic model using any material in your home. After learning the lesson, make a collage on Rutherford’s model of the atom using indigenous materials. V. REMARK VI. REFLECTION