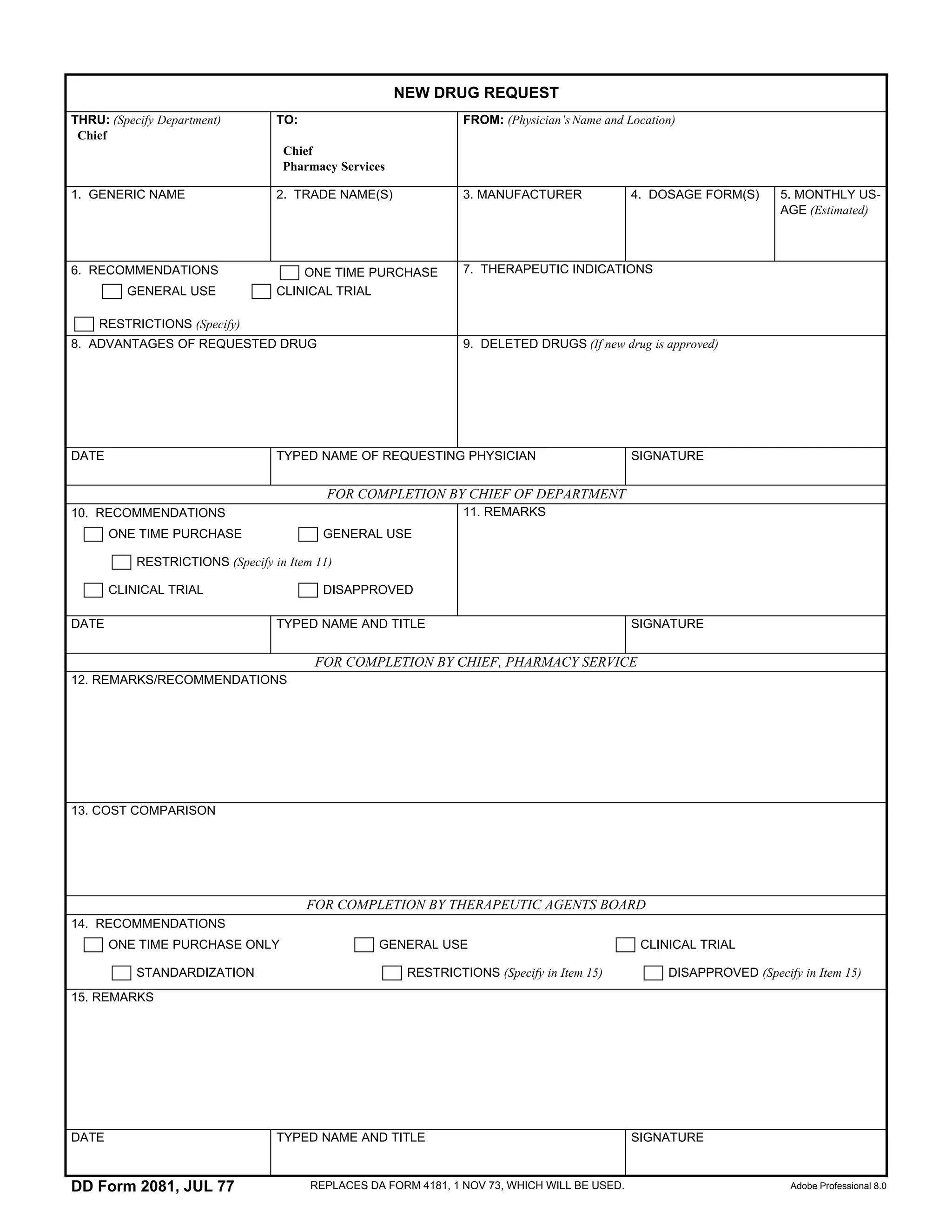
A physician is requesting approval for a new drug. The request form provides information about the generic and trade names, manufacturer, dosage form, estimated monthly usage, therapeutic indications, advantages over deleted drugs. Space is included for recommendations and remarks from the chief of department, chief of pharmacy services, therapeutic agents board regarding approval for a one time purchase, general use, restrictions, or as part of a clinical trial.