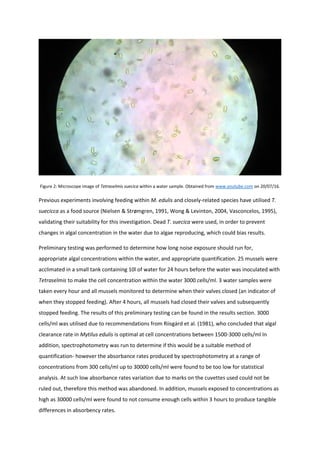
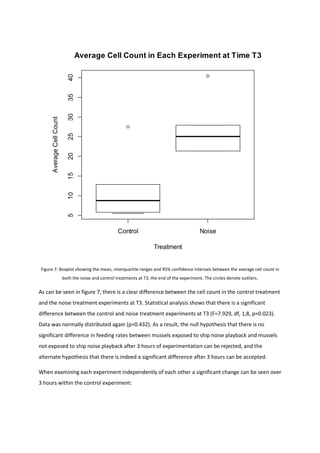
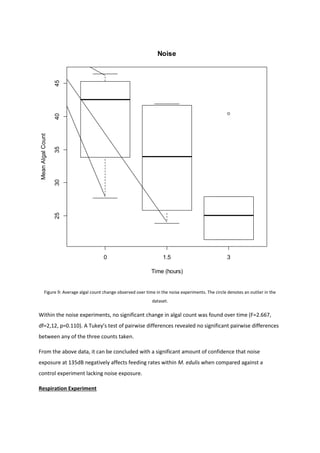
This document summarizes a project report on the effects of anthropogenic noise pollution on marine species physiology and behavior. It introduces the need to understand noise pollution impacts to help create effective legislation protecting marine environments under criteria like the EU Marine Strategy Framework Directive. While chemical pollution is well-studied, noise pollution effects are less understood. The document reviews noise propagation properties in water and potential impacts on various taxa to motivate further research on a commercially important shellfish, Mytilus Edulis.