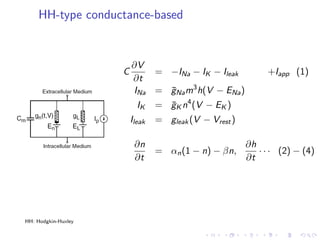
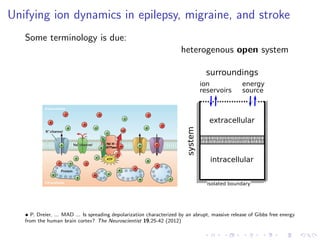
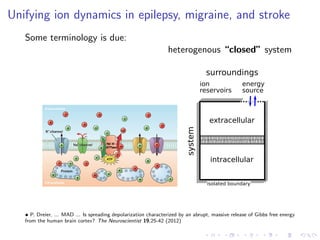
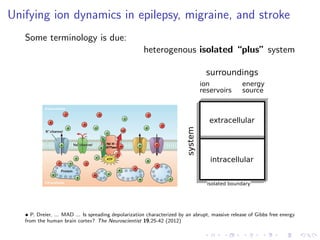
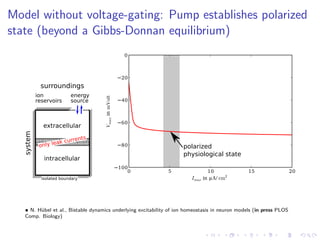
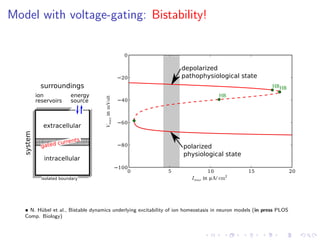
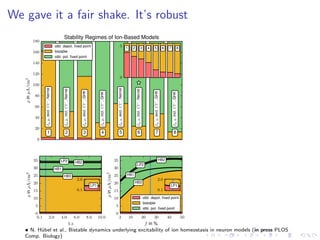
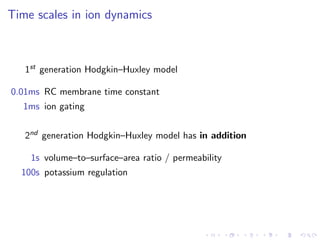
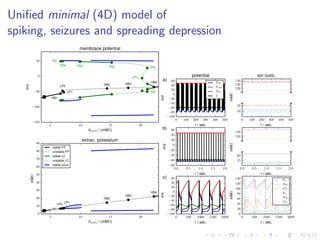
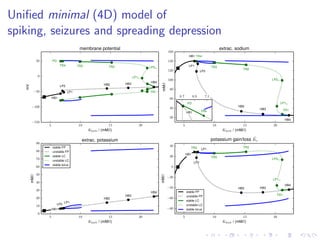
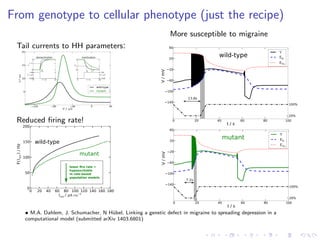
The document presents a unifying framework for modeling ion dynamics in the brain that can explain phenomena seen in epilepsy, migraine, and stroke. It introduces a second generation of Hodgkin-Huxley type models that account for ion concentrations and pumping/buffering dynamics in addition to membrane voltage dynamics. These models exhibit bistability between polarized and depolarized states, depending on parameters. The framework aims to provide a common mechanistic explanation for different neurological disorders based on shifts between stable states induced by genetic or external factors.





![From HH-type conductance-based to
conductance- & ion-based models (2nd
generation model)
3Na+
2K+
K+
Na+
K+
K+
Na+
Extracellular Space
Bath/Vasculature
Neuron
Cl
Diffusion
Cl
-
-
C
∂V
∂t
= −INa − IK − Ileak−Ipump+Iapp (1)
INa = ¯gNam3
h(V − ENa)
IK = ¯gK n4
(V − EK )
Ileak = gleak(V − Vrest)
∂n
∂t
= αn(1 − n) − βn,
∂h
∂t
· · · (2) − (4)
∂[ion]e
∂t
= −
A
FVolo
Iion
∂[ion]i
∂t
=
A
FVoli
Iion (5) − · · ·
HH: Hodgkin-Huxley](https://image.slidesharecdn.com/dahlemdpg2014-140402085123-phpapp02/85/From-epilepsy-to-migraine-to-stroke-A-unifying-framework-9-320.jpg)
![From HH-type conductance-based to
conductance- & ion-based models (2nd
generation model)
ion
reservoirs
isolated boundary
system
surroundings
energy
source
extracellular
intracellular
C
∂V
∂t
= −INa − IK − Ileak−Ipump+Iapp (1)
INa = ¯gNam3
h(V − ENa)
IK = ¯gK n4
(V − EK )
Ileak = gleak(V − Vrest)
∂n
∂t
= αn(1 − n) − βn,
∂h
∂t
· · · (2) − (4)
∂[ion]e
∂t
= −
A
FVolo
Iion
∂[ion]i
∂t
=
A
FVoli
Iion (5) − · · ·
HH: Hodgkin-Huxley](https://image.slidesharecdn.com/dahlemdpg2014-140402085123-phpapp02/85/From-epilepsy-to-migraine-to-stroke-A-unifying-framework-10-320.jpg)
![Choices: Current and pump equations, ions, ...
Two pump types
Iion,pumped,1([K]o, [Na]i ) = Imax 1 +
KmK
[K]o
−2
1 +
KmNa
[Na]i
−3
Iion,pumped,2([K]o, [Na]i ) = Imax
1
1 + e(25−[Na]i /3)
1
1 + e(5.5−[K]o)
HH current or GHK currents
Iion = gion(V − Eion)
Iion = V αF Pion
[ion]i − [ion]oe−αV
1 − e−αV
With or without chloride dynamics
d[Cl−]
dt
= ... or 0](https://image.slidesharecdn.com/dahlemdpg2014-140402085123-phpapp02/85/From-epilepsy-to-migraine-to-stroke-A-unifying-framework-19-320.jpg)
![HH 2nd
-generation “closed” systems are bistable
C
∂V
∂t
= −
ion
(Iion,gated + Iion,pumped )
current, pump, and gating equations . . .
∂[K+]e
∂t
=
A
FVole
(IK+,gated + IK+,pumped )
ion
reservoirs
isolated boundary
system
surroundings
energy
source
extracellular
intracellular
0 5 10 15 20
Imax in µA/ cm2
100
80
60
40
20
0
VmaxinmVolt
HB
HBHB
polarized
physiological state
depolarized
pathophysiological state](https://image.slidesharecdn.com/dahlemdpg2014-140402085123-phpapp02/85/From-epilepsy-to-migraine-to-stroke-A-unifying-framework-21-320.jpg)
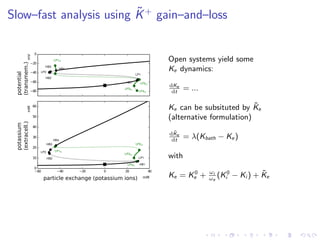
![Open system: Diffusion (buffering) of potassium
C
∂V
∂t
= −
ion
(Iion,gated + Iion,pumped )
fixed point!
= 0
current, pump, and gating equations . . .
∂[K+]e
∂t
=
A
FVole
(IK+,gated + IK+,pumped )+ λ([K+
]bath − [K+
]e)
buffer to bath
fixed point!
= 0
f.p.!
= 0
ion
reservoirs
isolated boundary
system
surroundings
energy
source
extracellular
intracellular
0 5 10 15 20
Imax in µA/ cm2
100
80
60
40
20
0
VmaxinmVolt
HB
HBHB
polarized
physiological state
depolarized
pathophysiological state](https://image.slidesharecdn.com/dahlemdpg2014-140402085123-phpapp02/85/From-epilepsy-to-migraine-to-stroke-A-unifying-framework-22-320.jpg)

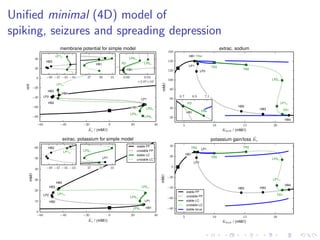
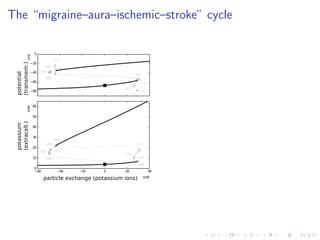
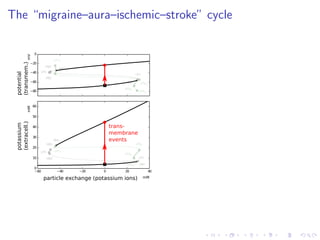
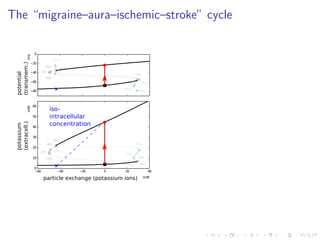
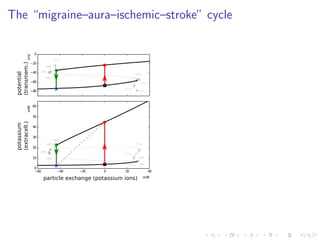
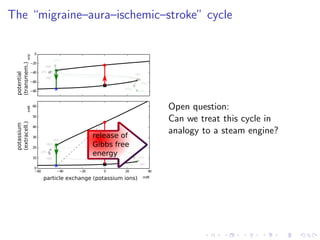
![The “ceiling level” of [K+
]e in seizure activity
−80
−60
−40
−20
0
HB1
LP1
HB2
LP2
HB3
HB4
LP1lc
lc
lc
LP4lc
potential
(transmem.)
−60 −40 −20 0 20 40
0
10
20
30
40
50
60
HB1
LP1HB2
LP2
HB3
HB4
LP1lc
LP4lc
particle exchange (potassium ions)
potassium
(extracell.)
mM
mMmV
LP2
LP3
LP2lc
LP3lc
seizure-like
activity
"ceiling level"](https://image.slidesharecdn.com/dahlemdpg2014-140402085123-phpapp02/85/From-epilepsy-to-migraine-to-stroke-A-unifying-framework-34-320.jpg)


![Conclusions
Including ion dynamics into a Hodghin-Huxley framework
yields slow quasiperodic dynamics:
important bifurcation parameter is gain–and–loss of ions,
explain the “ceiling level” of [K+
]e in seizure activity,
explain the “migraine–aura–ischemic–stroke” contiuum.
No synaptic currents needed for slow dynamics, in particular,
no metabotropic receptor that acts through a secondary
messenger, like GABAB.
Remark : Ultra–slow (or near–DC (direct current)) activity
that cannot be observed by electroencephalography (EEG),
because it is susceptible to uncontrollable artifacts such as
changes in the resistance of the dura.
However : subdural electrode recordings provided recently
direct and unequivocal evidence that such dynamics occurs in
abundance in people with structural brain damage.](https://image.slidesharecdn.com/dahlemdpg2014-140402085123-phpapp02/85/From-epilepsy-to-migraine-to-stroke-A-unifying-framework-38-320.jpg)
