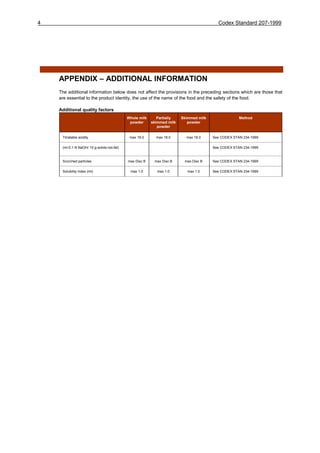
This document contains the Codex Standard for milk powders and cream powder. It establishes standards for various milk powders including whole milk powder, partly skimmed milk powder, skimmed milk powder, and cream powder. It defines the scope, describes the products, sets requirements for composition, quality factors, food additives, contaminants, hygiene, labeling, sampling and methods of analysis. The standard aims to protect consumer health, ensure fair practices in trade, and promote coordination of food standards internationally.