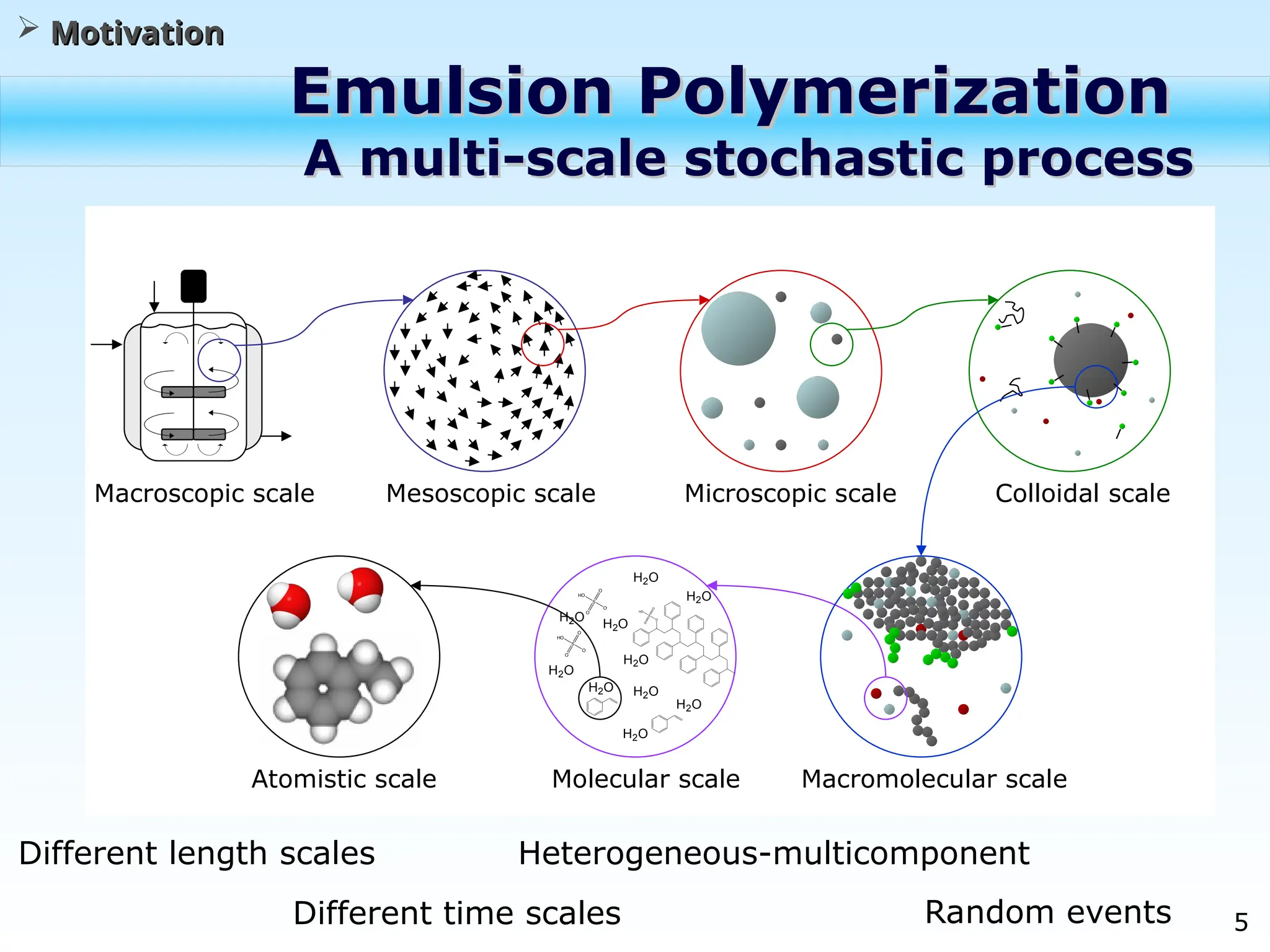
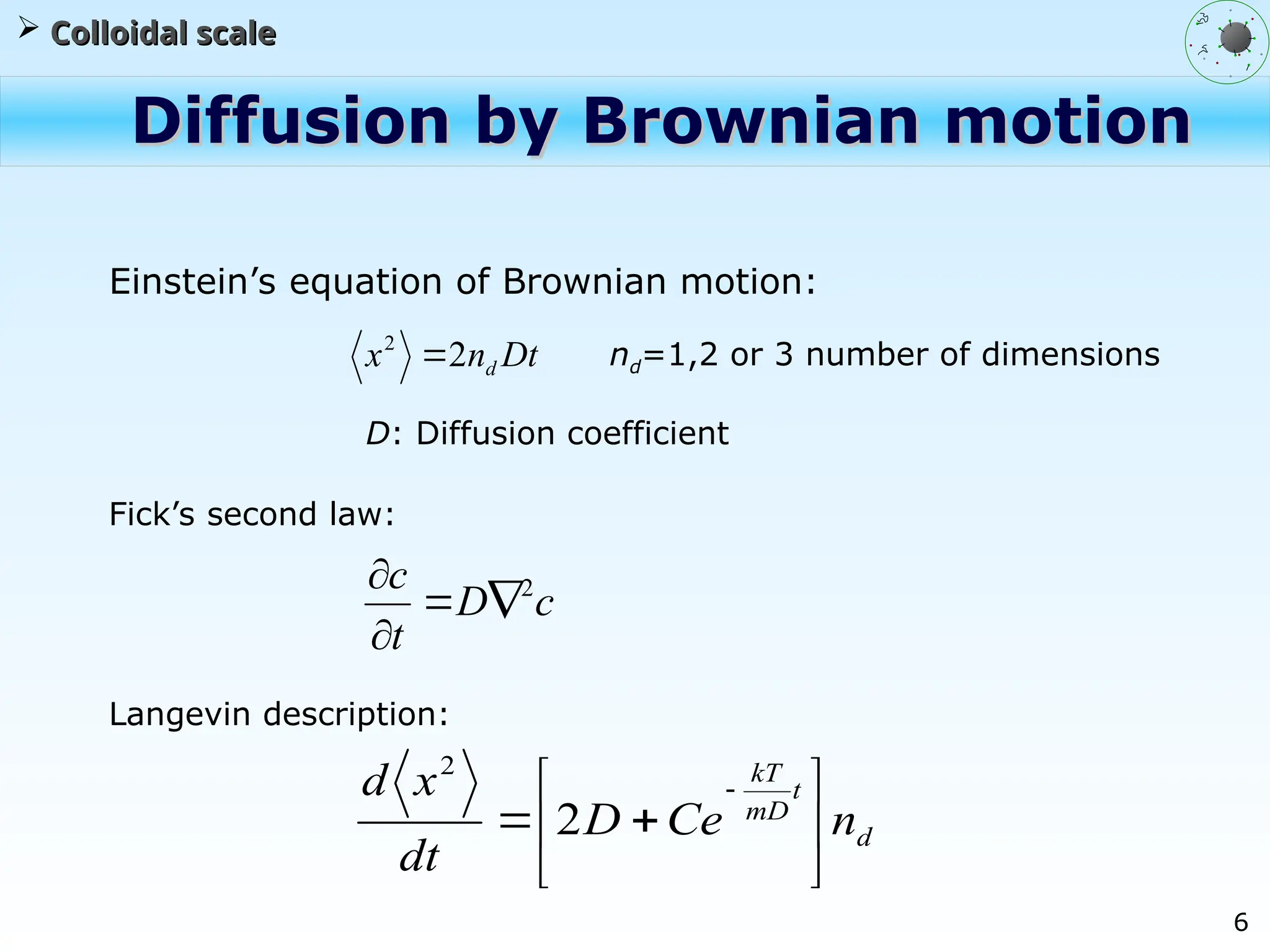
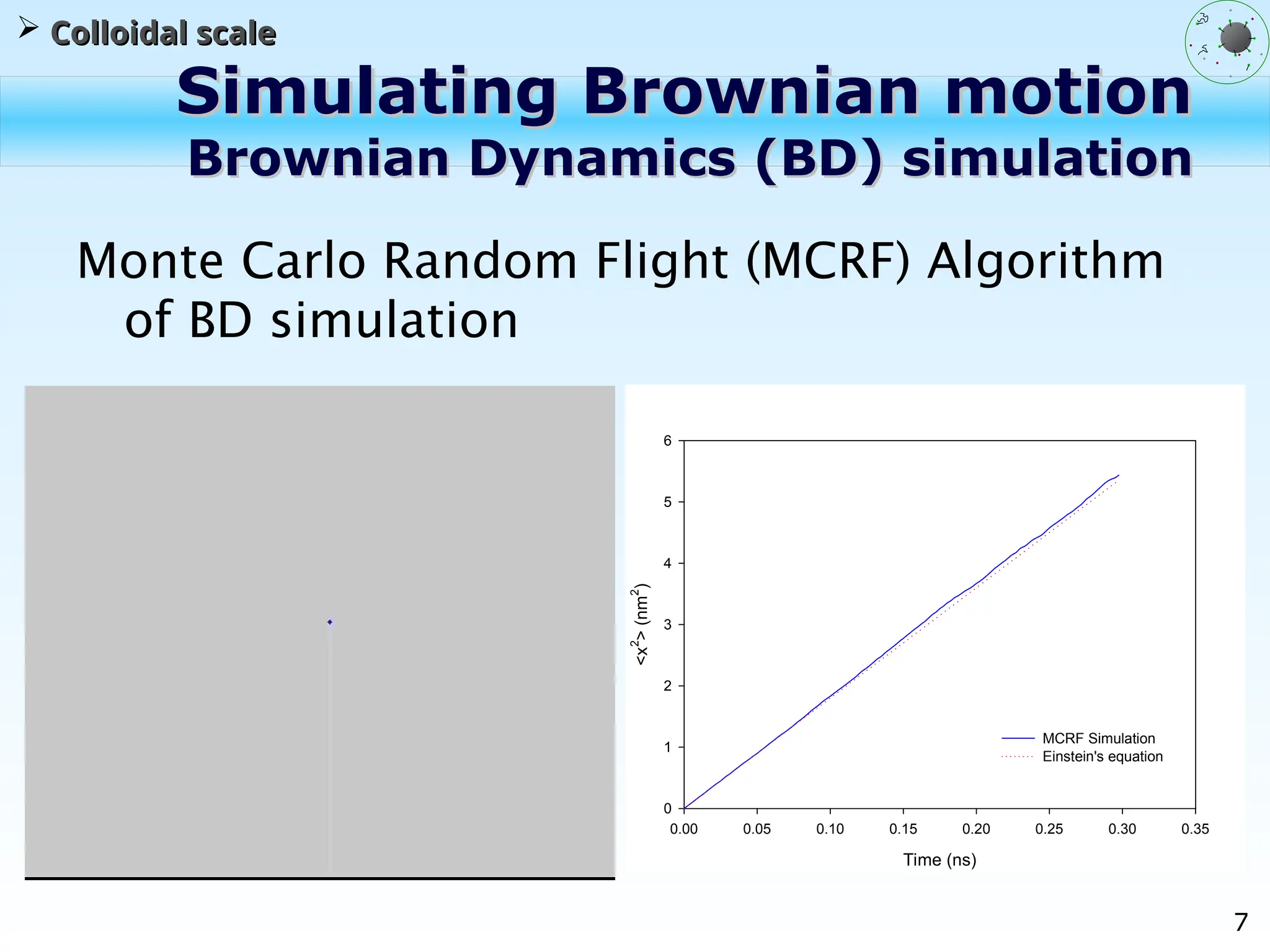
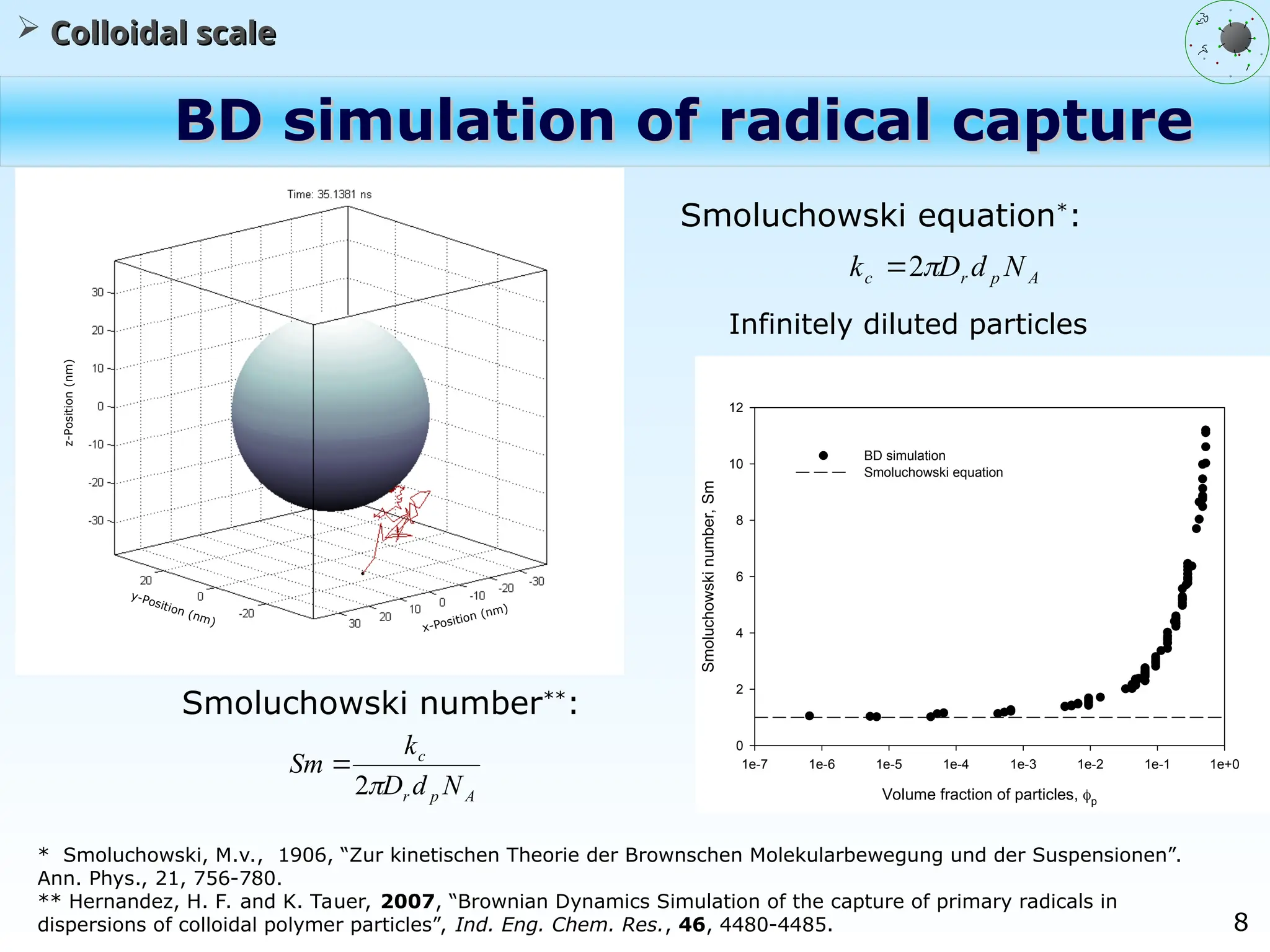
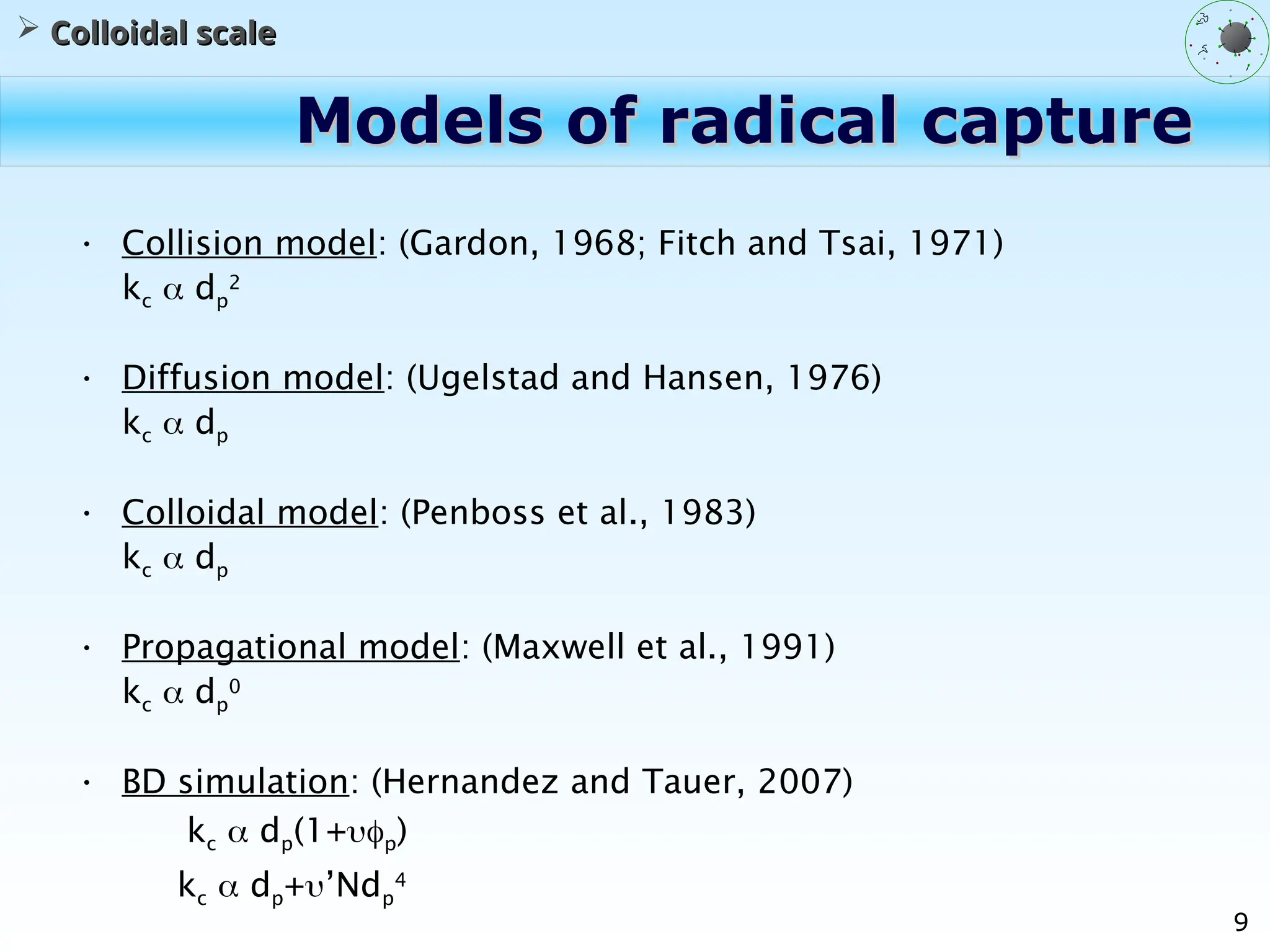
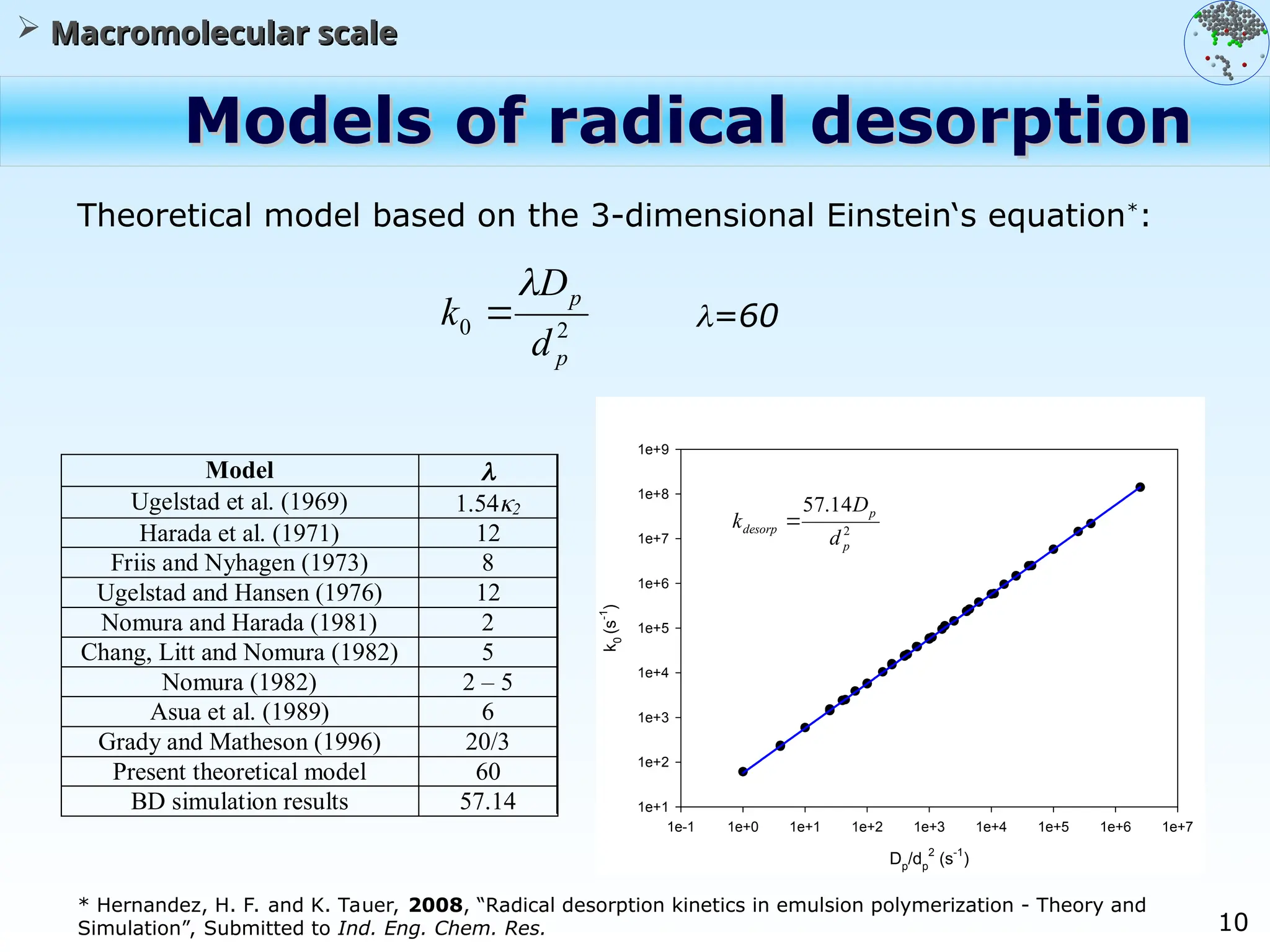
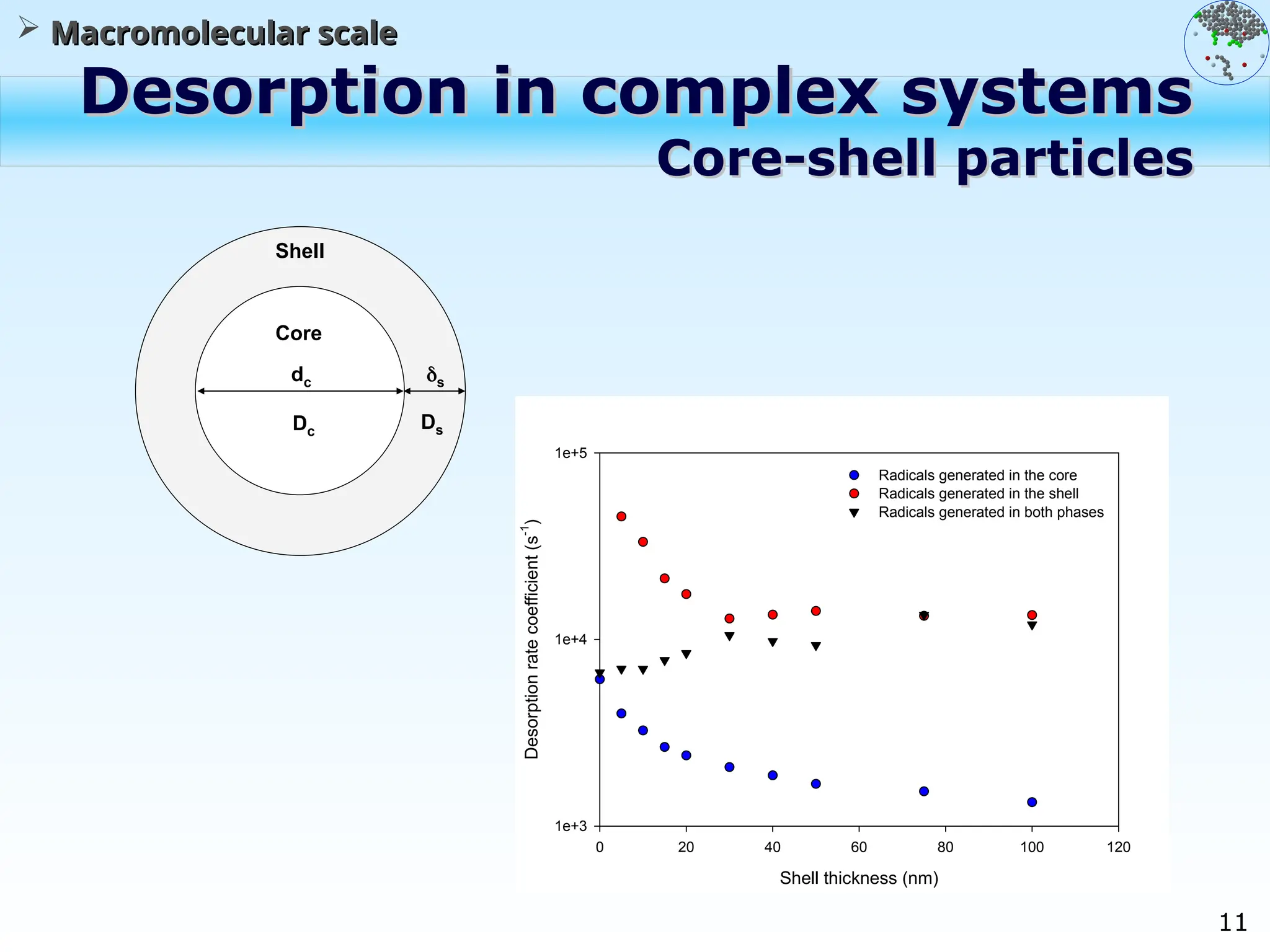
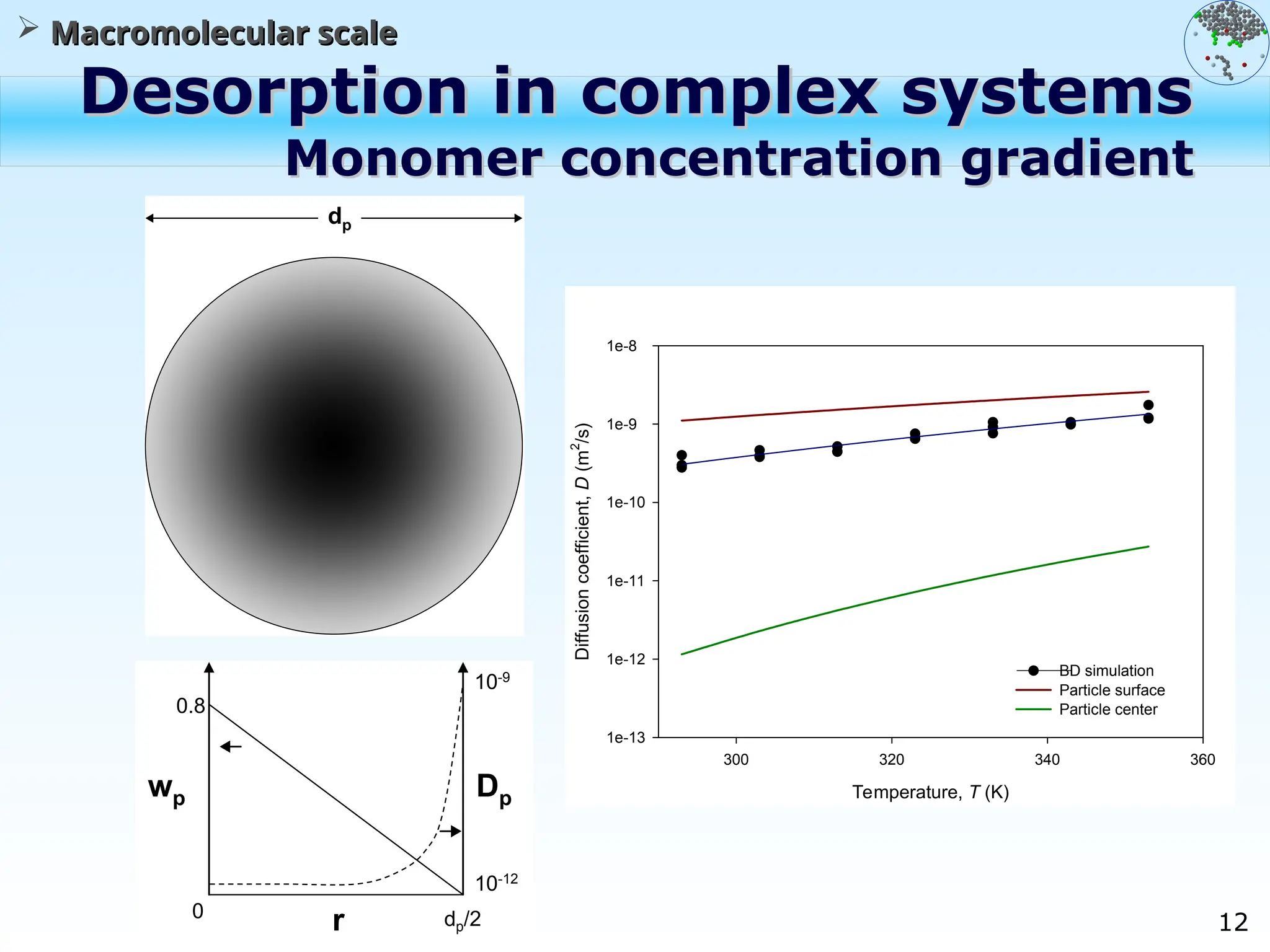
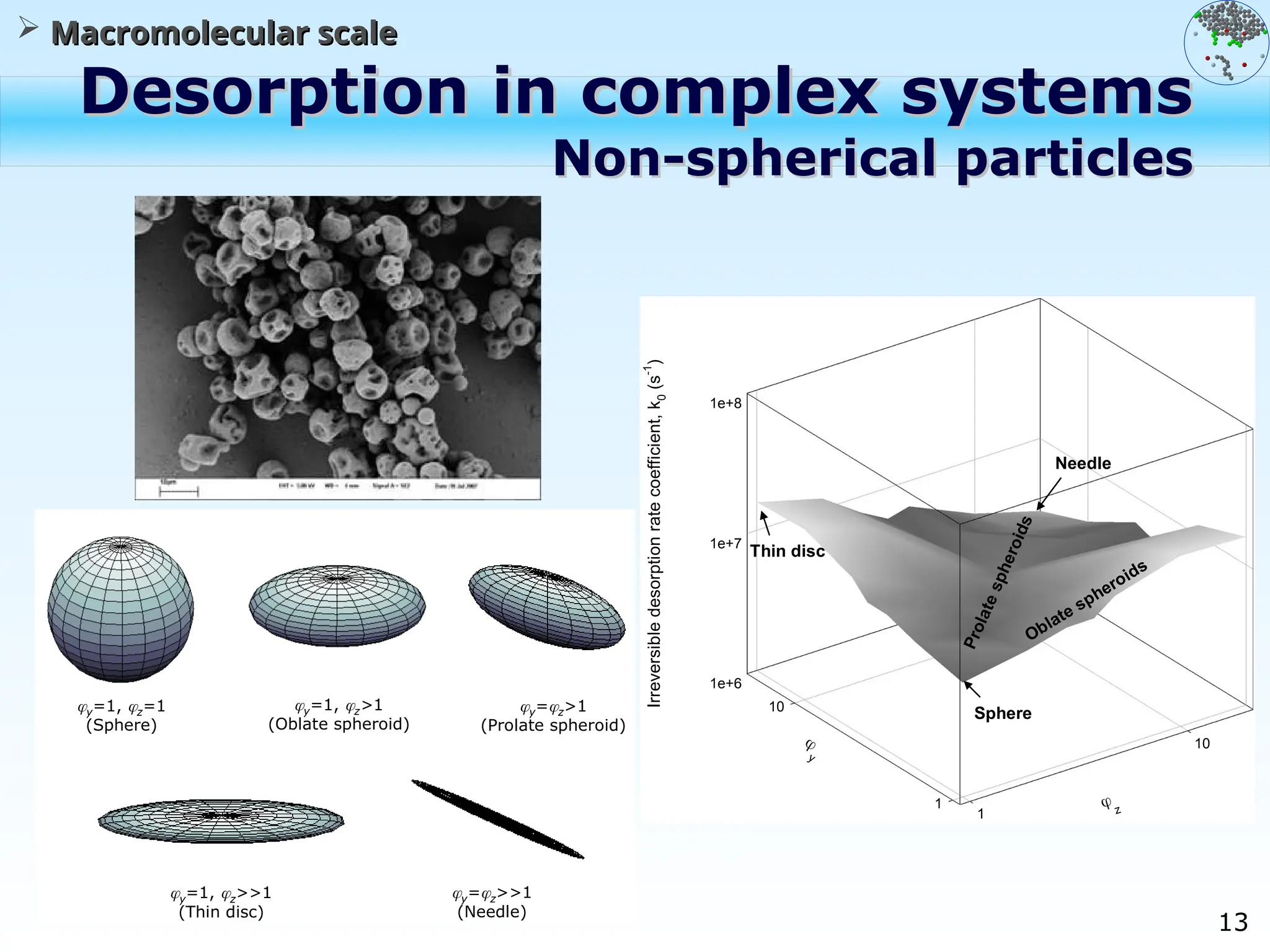
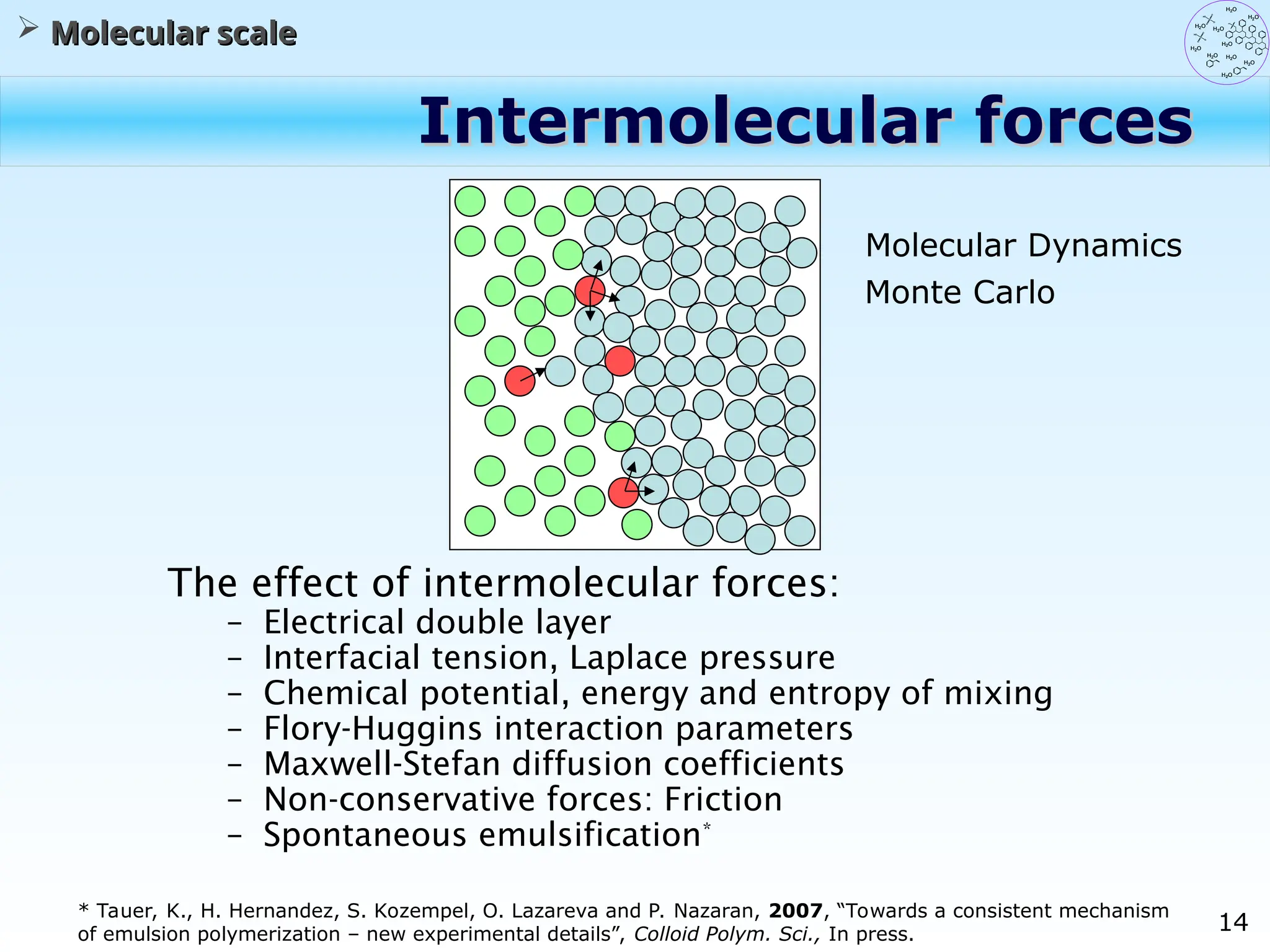
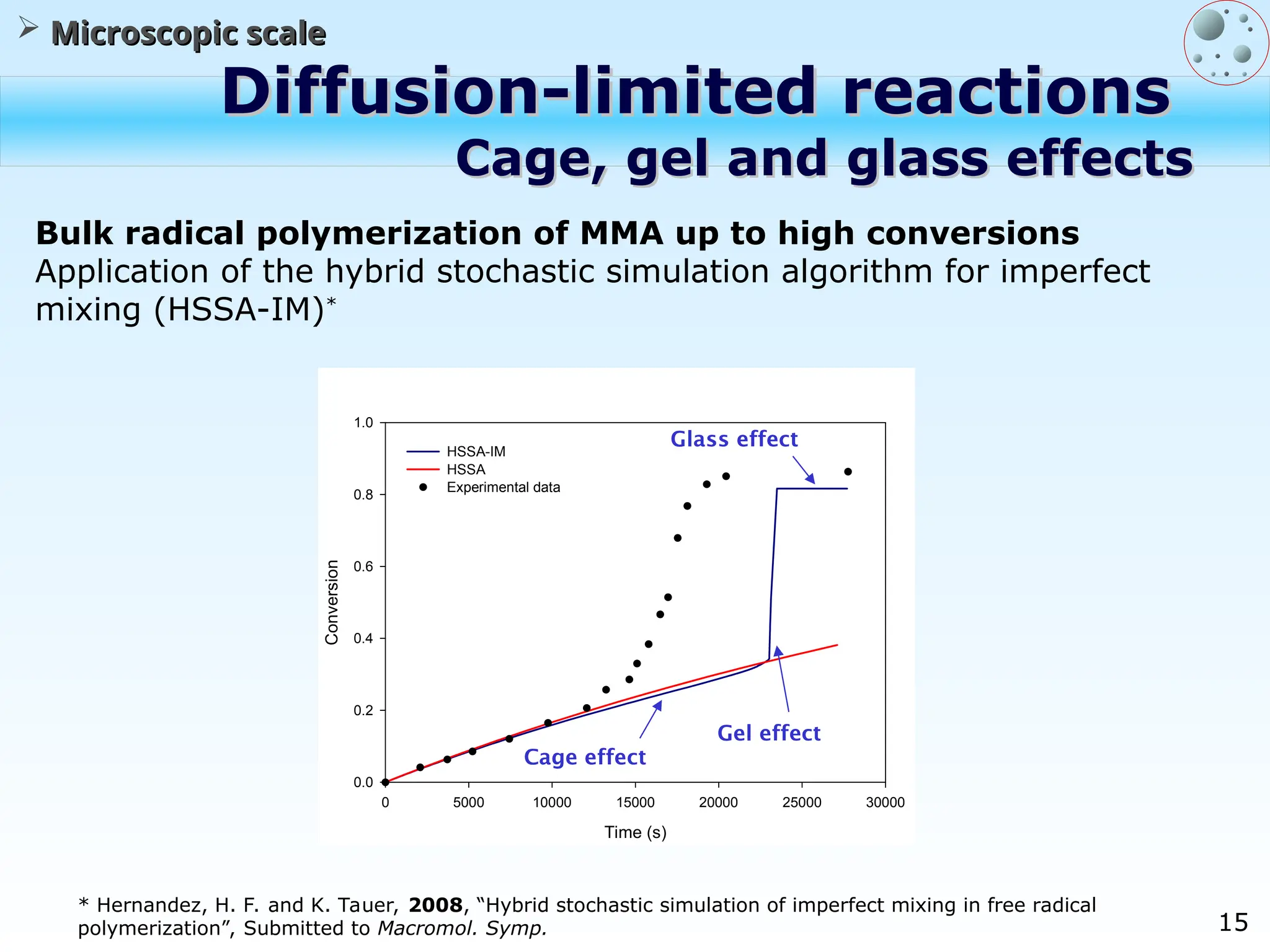
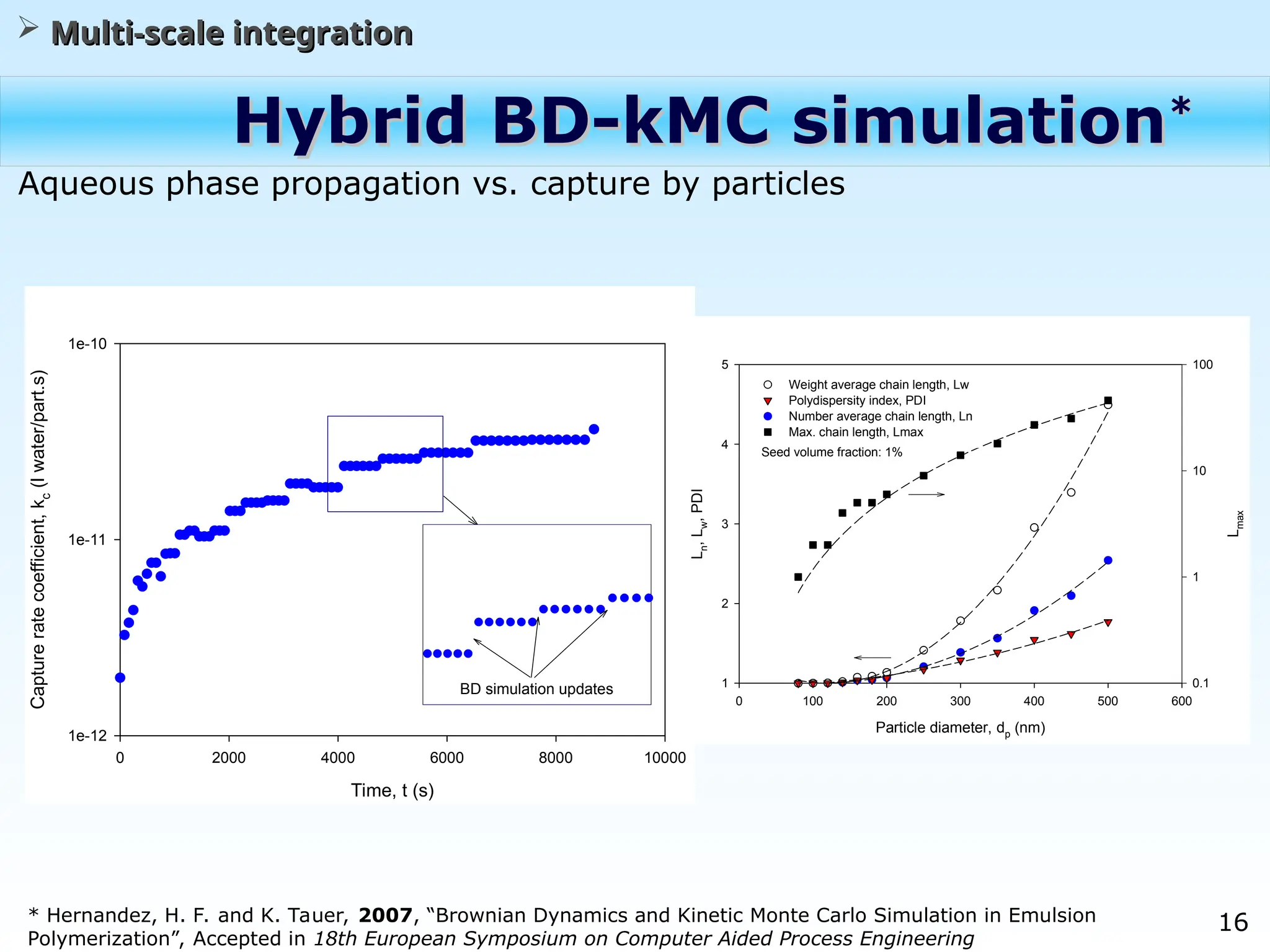
The document discusses the multiscale stochastic modeling of emulsion polymerization, highlighting the current gaps in understanding and the need for better experimental methods and models. It outlines various scales of analysis—from colloidal to macromolecular—and emphasizes the significance of integrating these scales for a comprehensive understanding of the polymerization process. Conclusively, it asserts that improving modeling and simulation across these scales is crucial for advancing knowledge in emulsion polymerization and related processes.