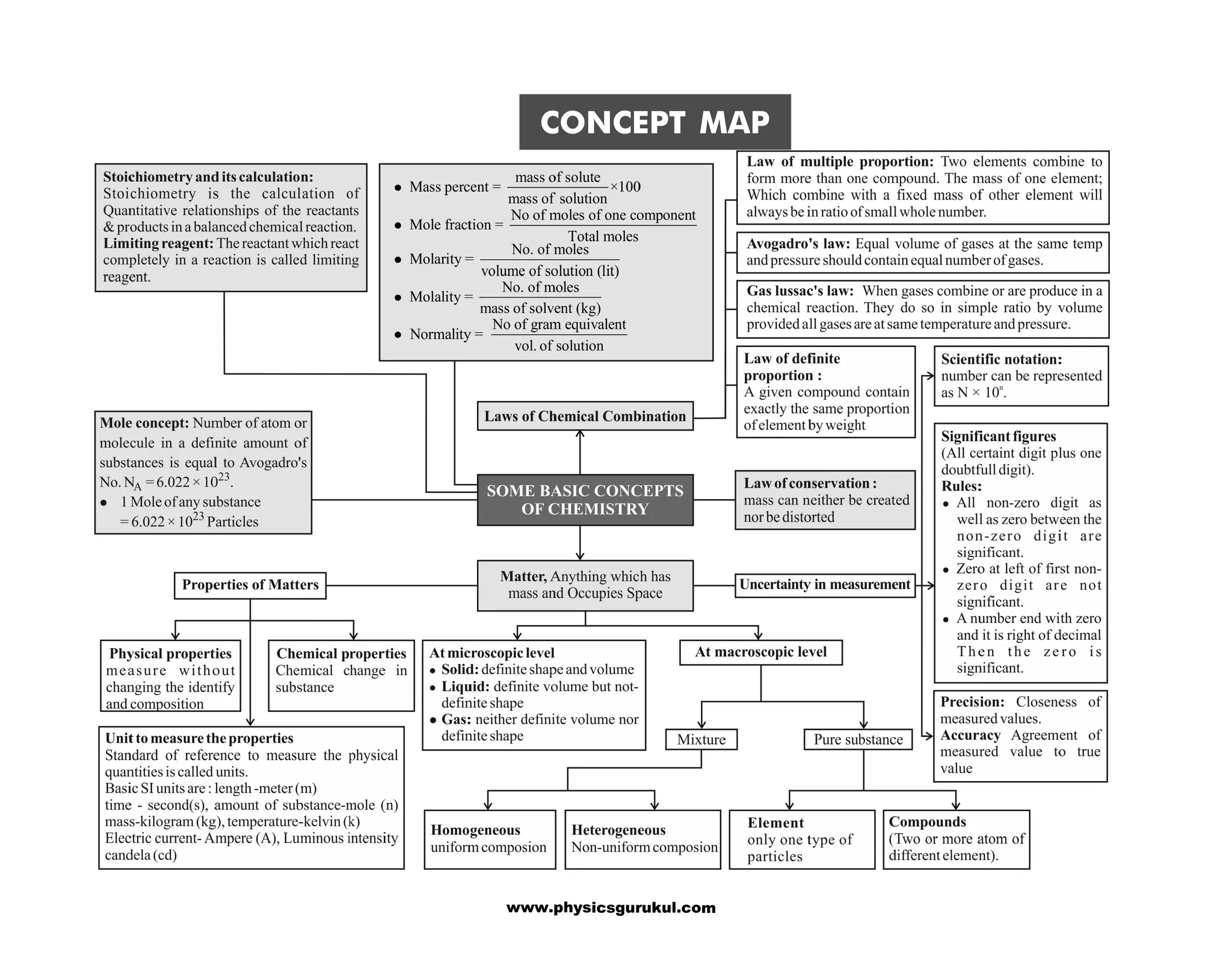
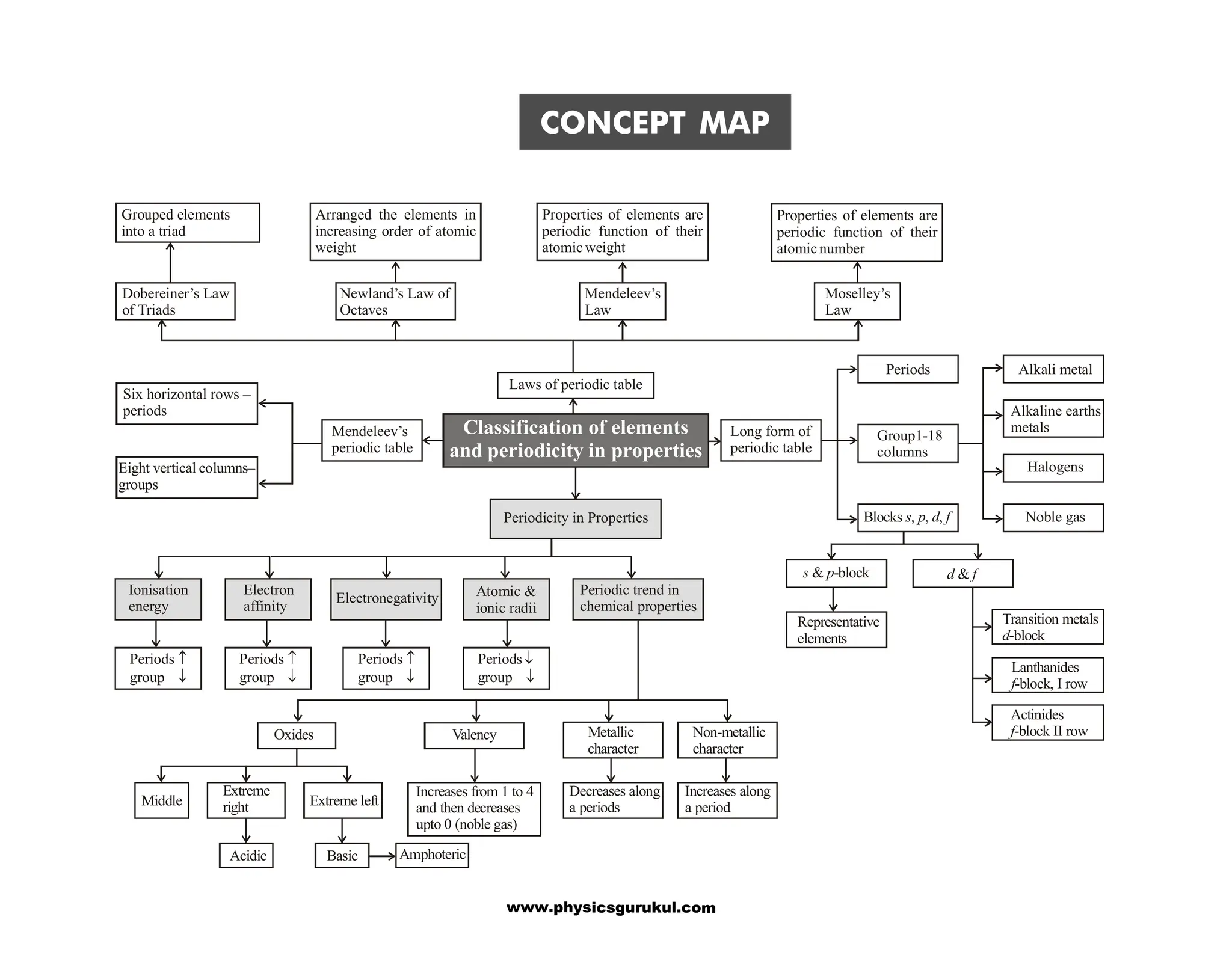
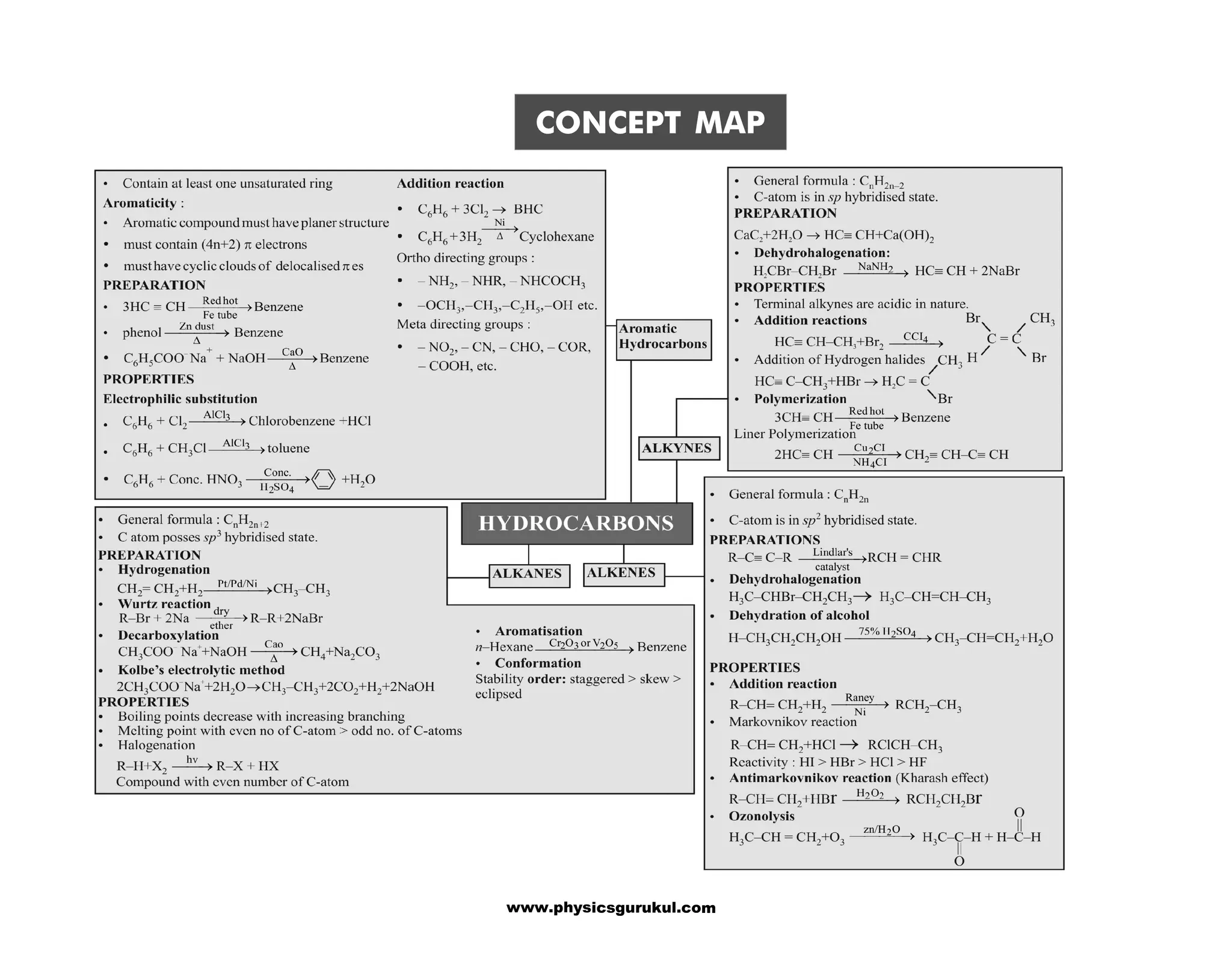
The document is a concept map covering essential chemistry topics for class 11, tailored for JEE and NEET preparation, including fundamental concepts such as the structure of the atom, periodic properties, chemical bonding, and thermodynamics. It outlines various concepts such as periodicity in properties, hydrogen's characteristics, and the properties of water and hydrides. Additionally, it discusses the preparation, properties, and uses of key chemical substances like dihydrogen and hydrogen peroxide.