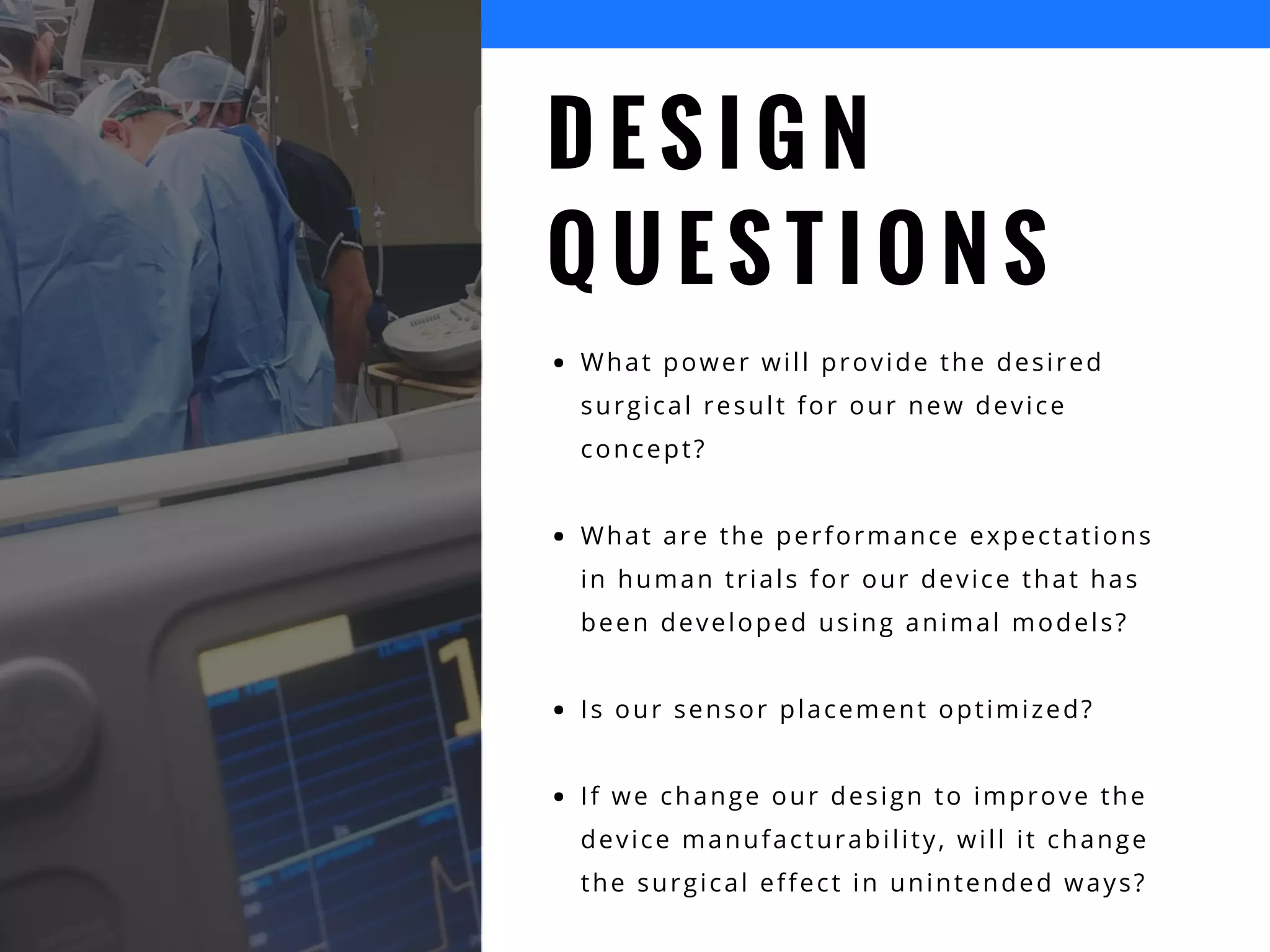
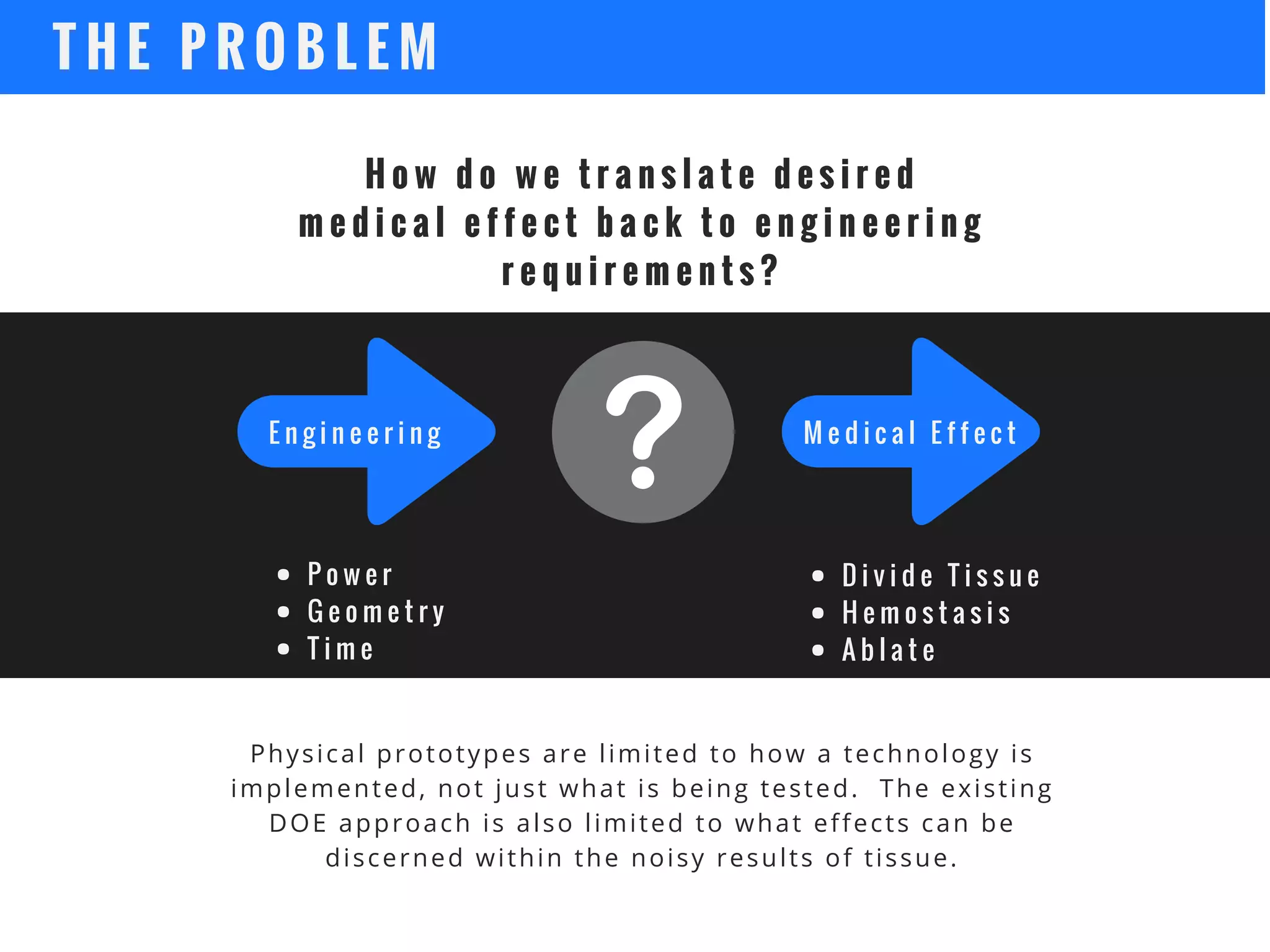
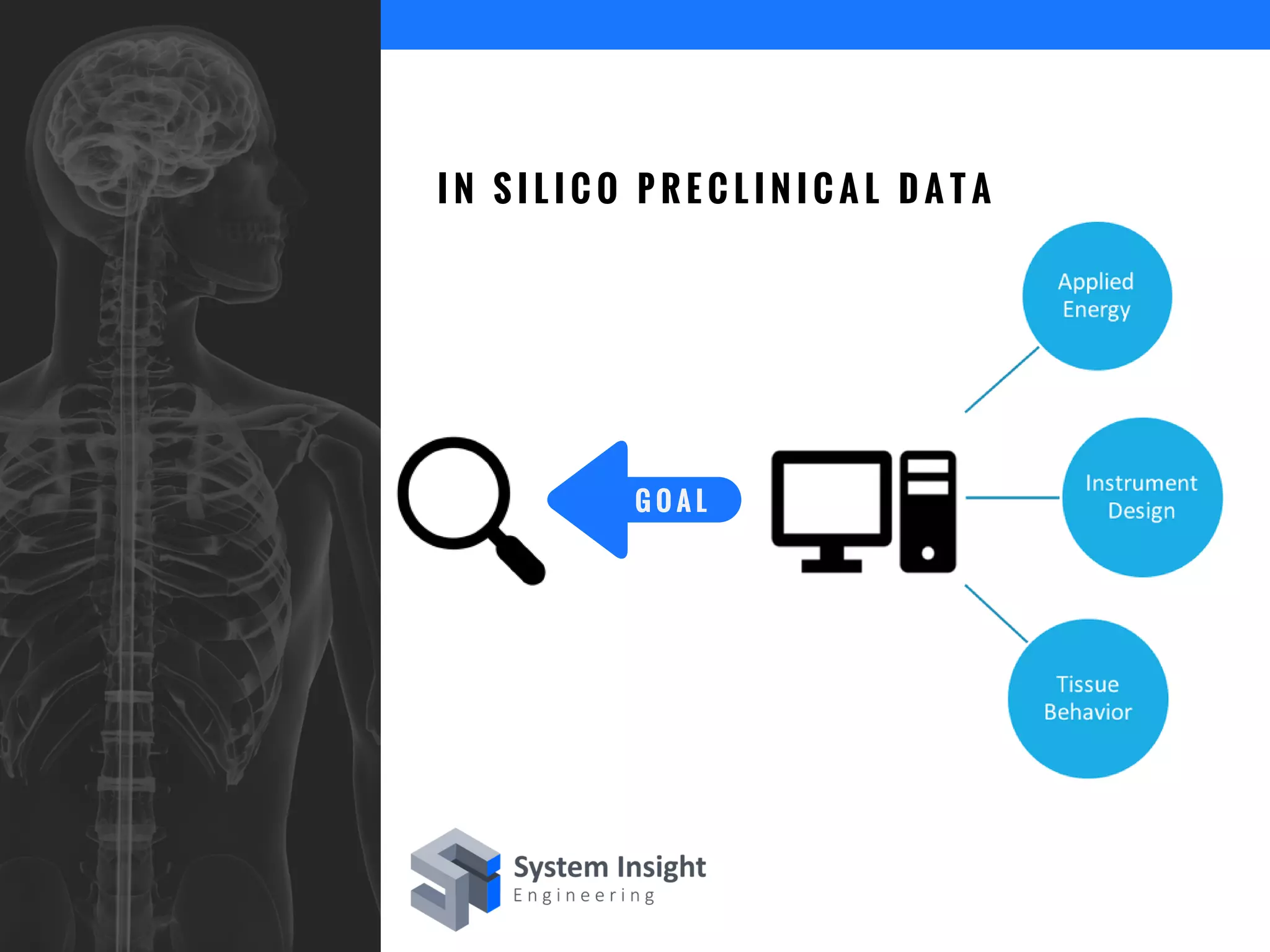
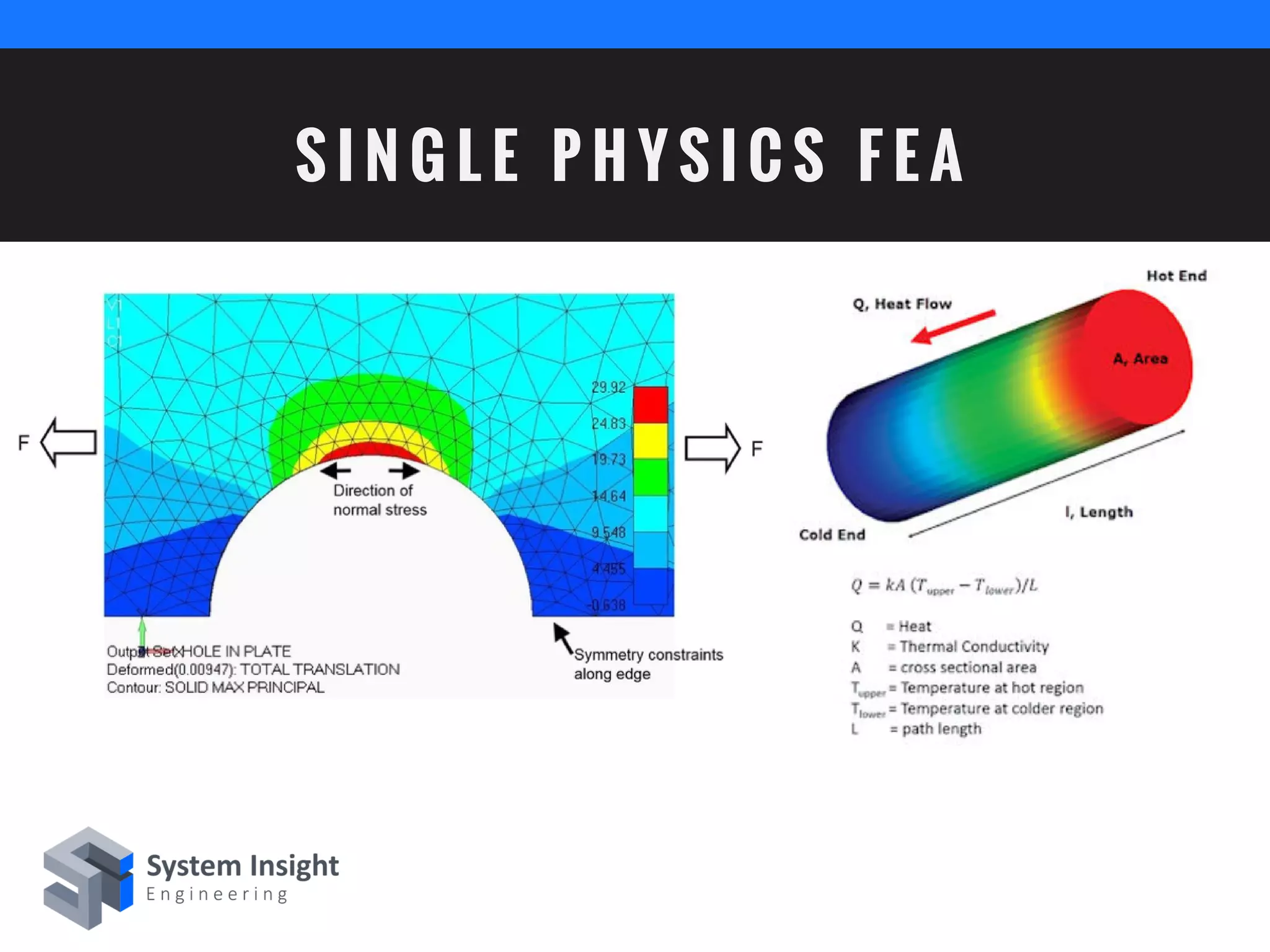
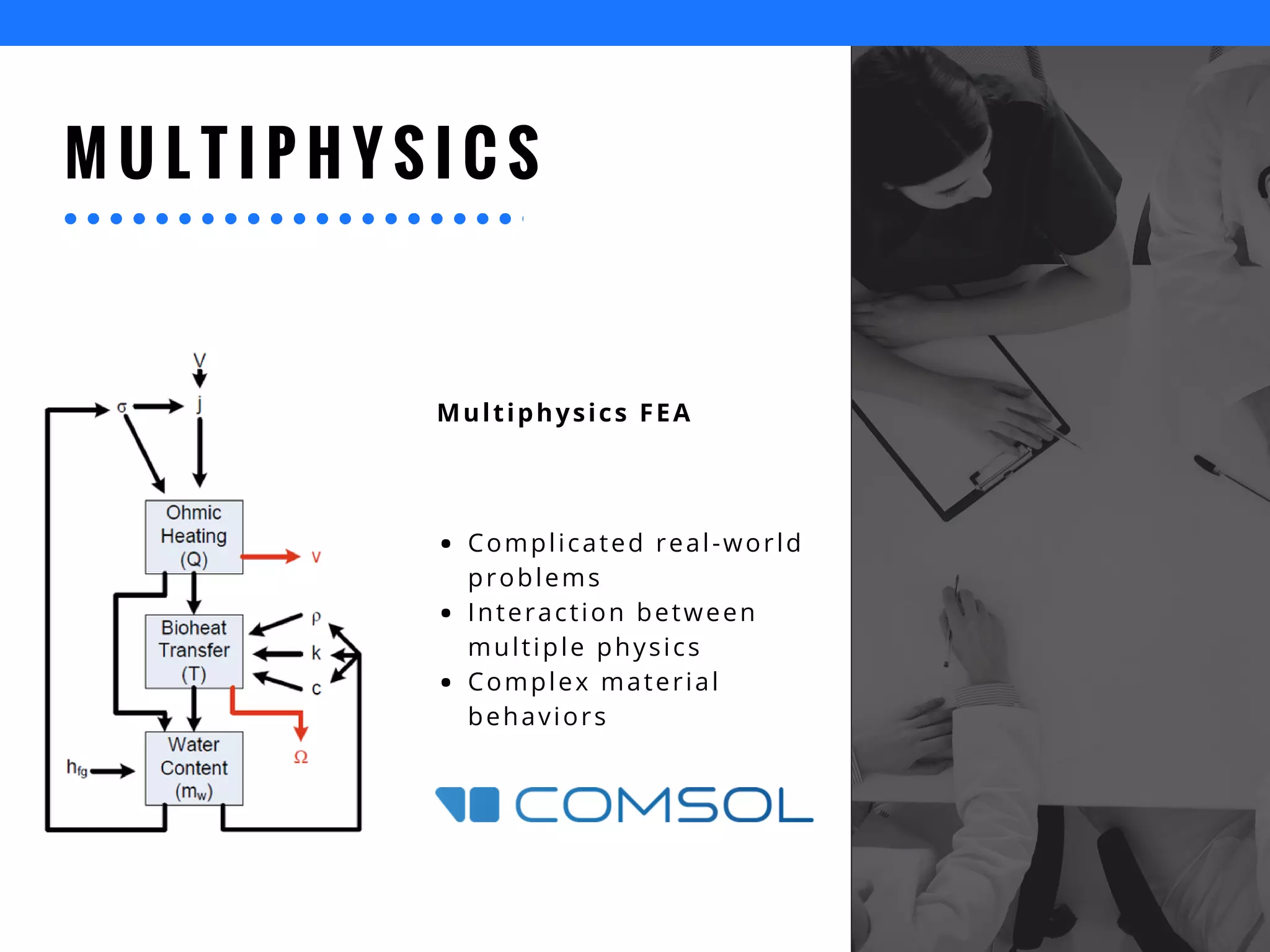
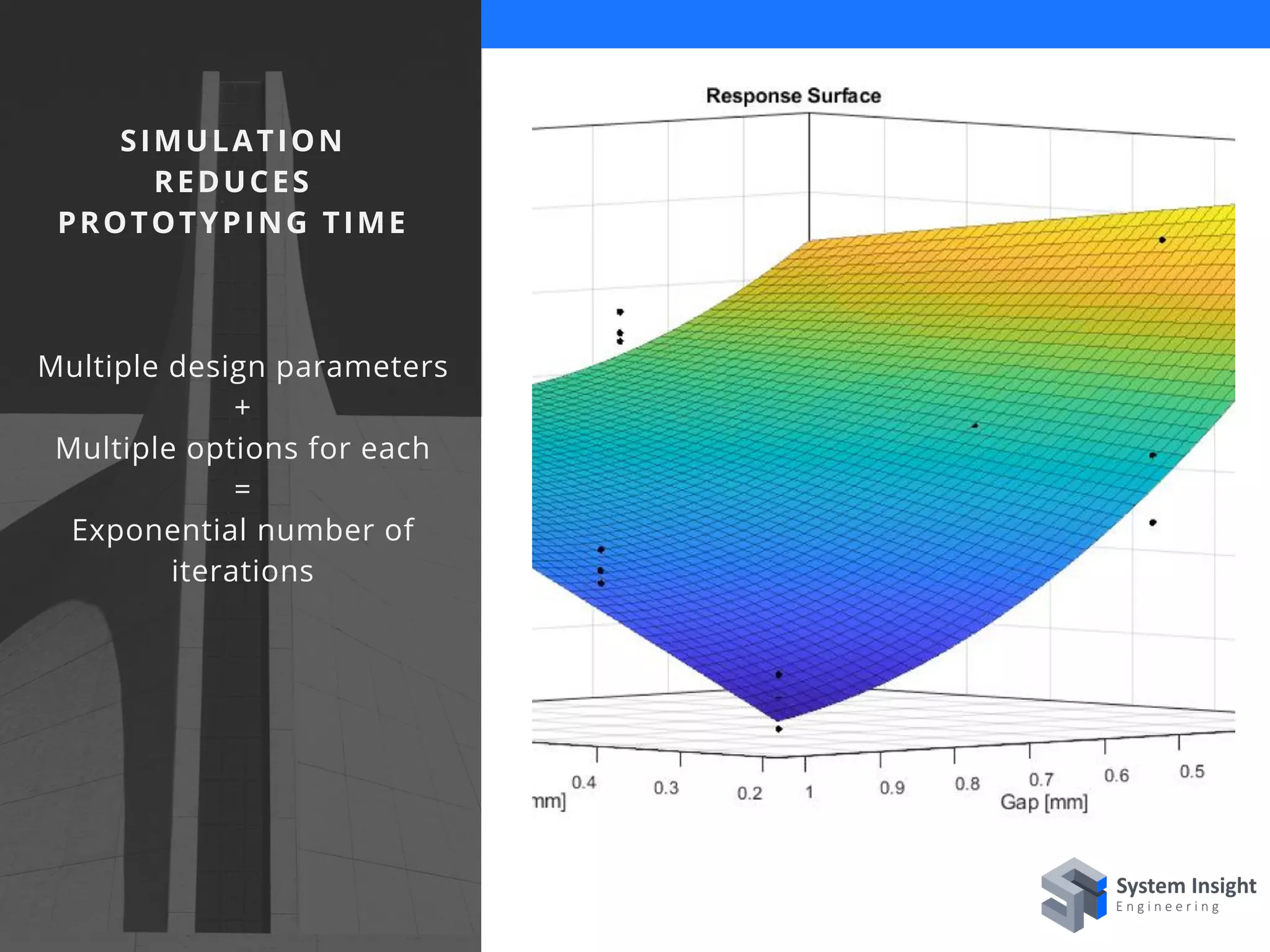
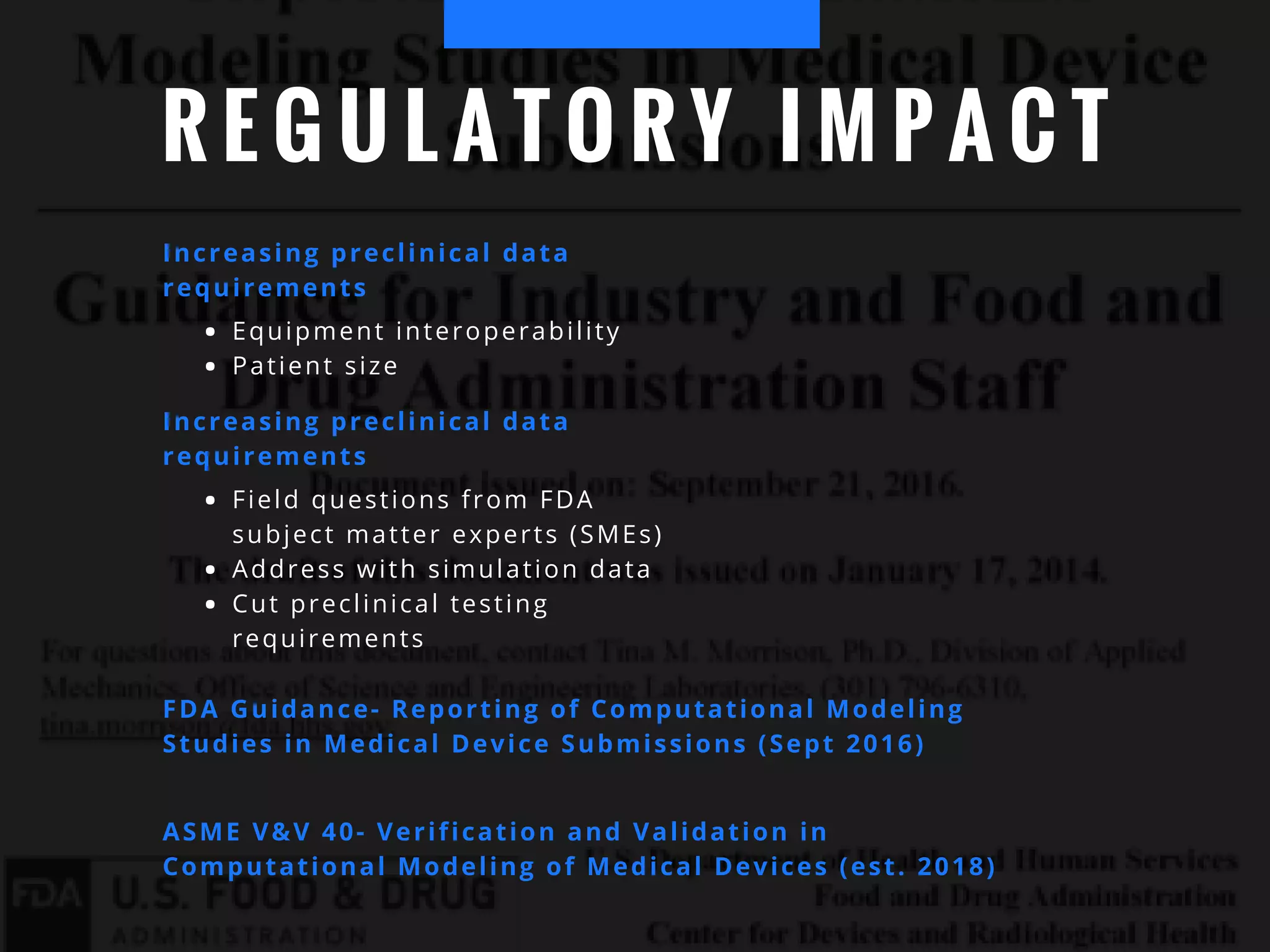
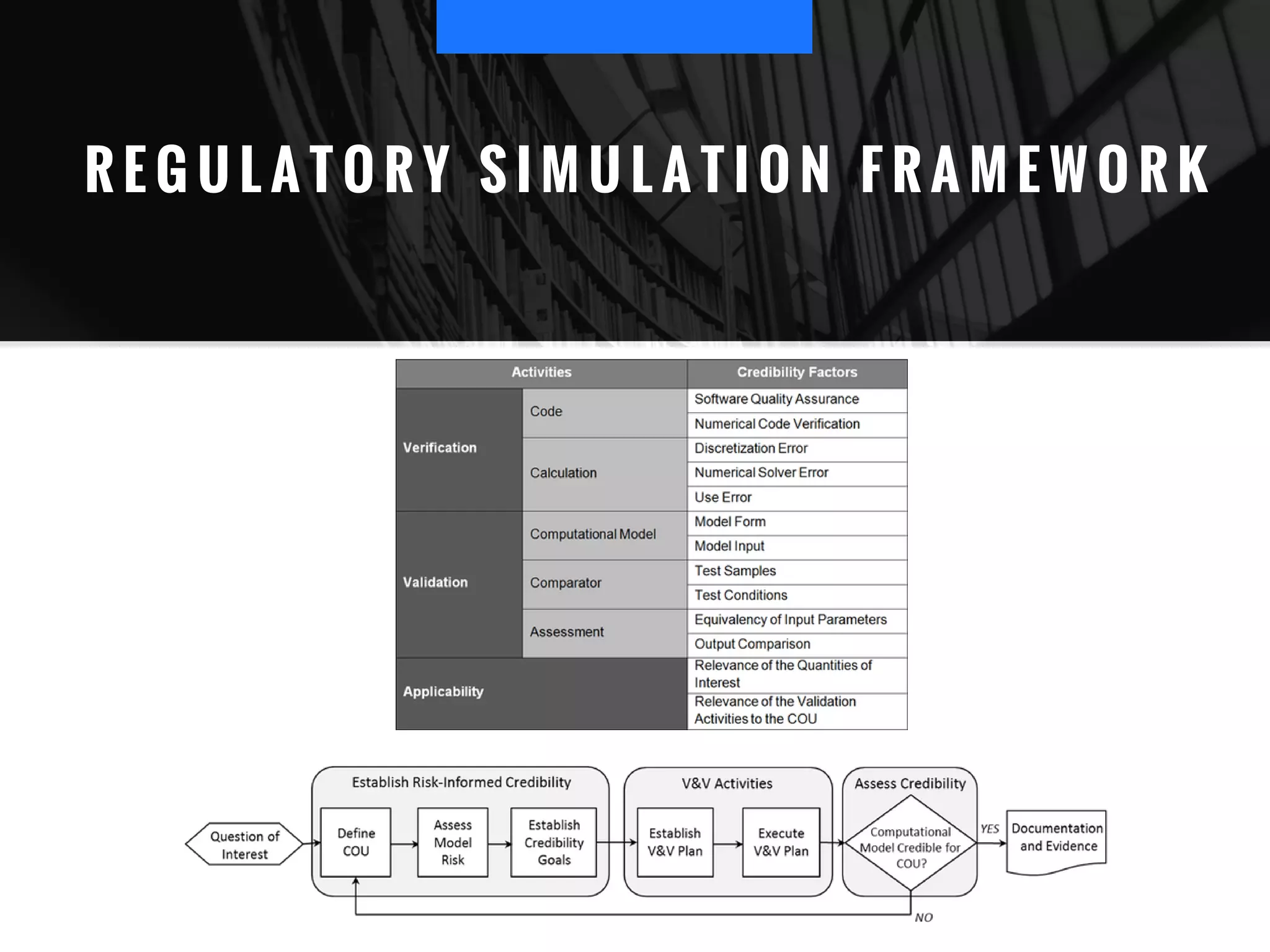
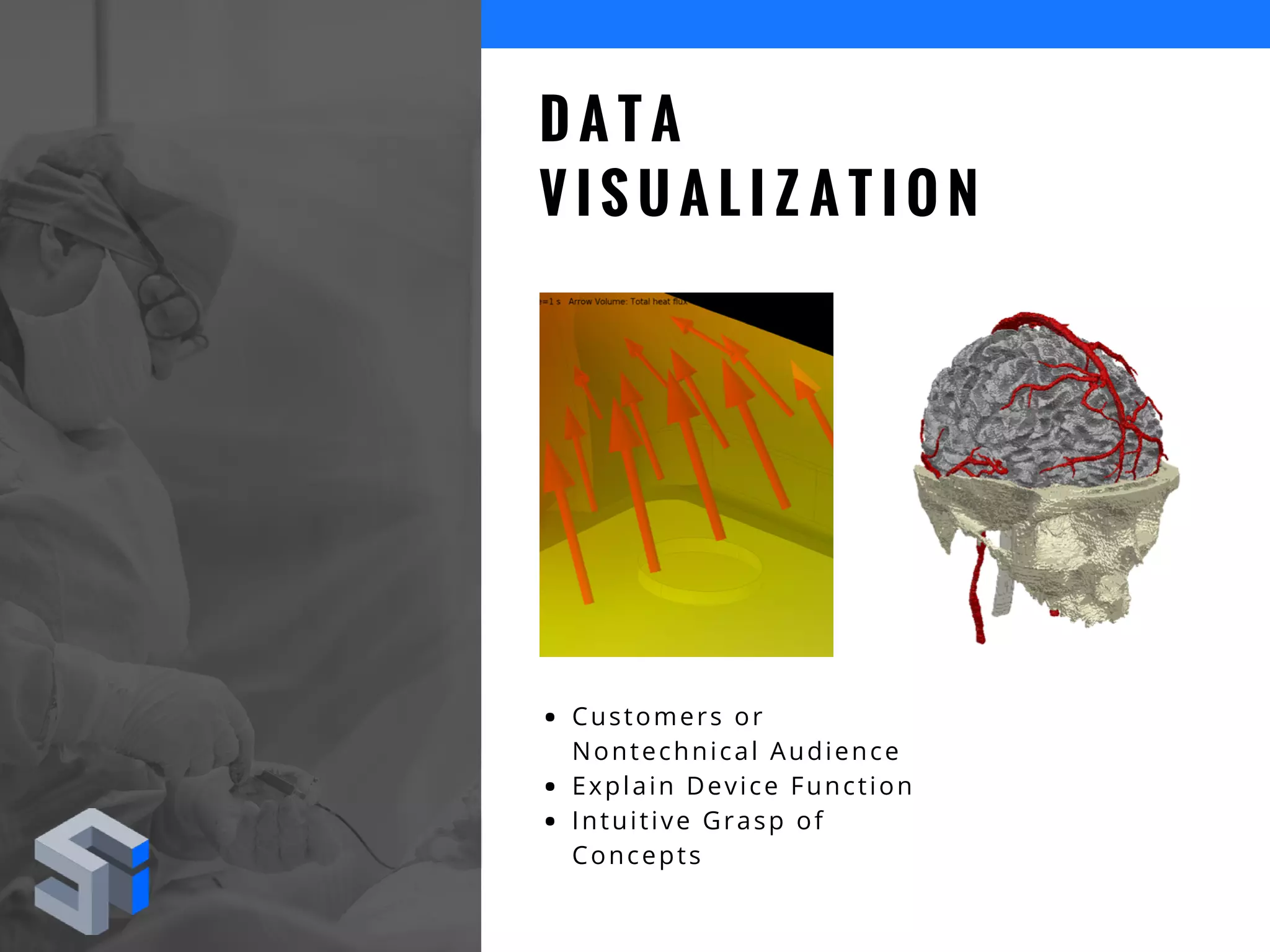
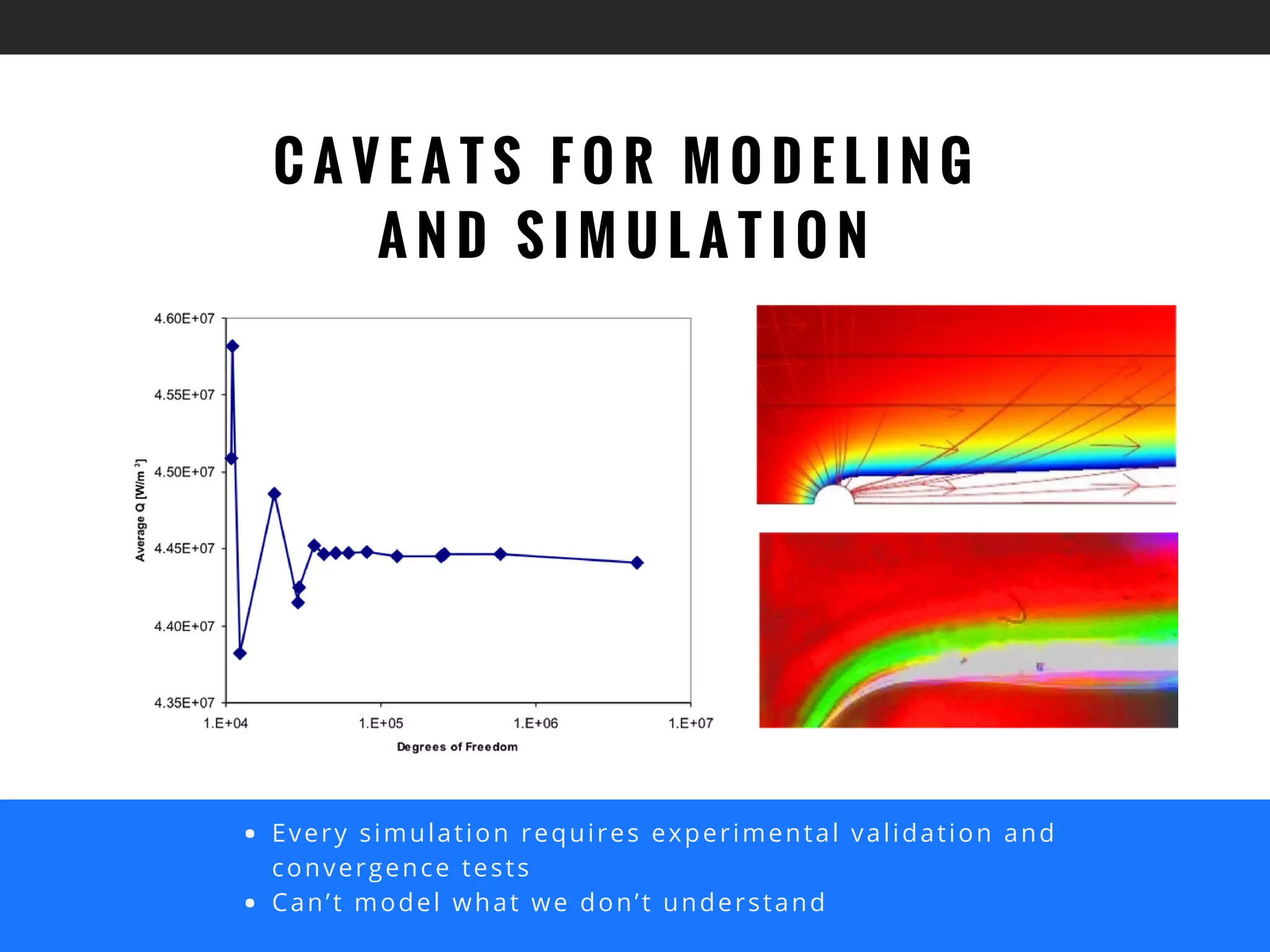
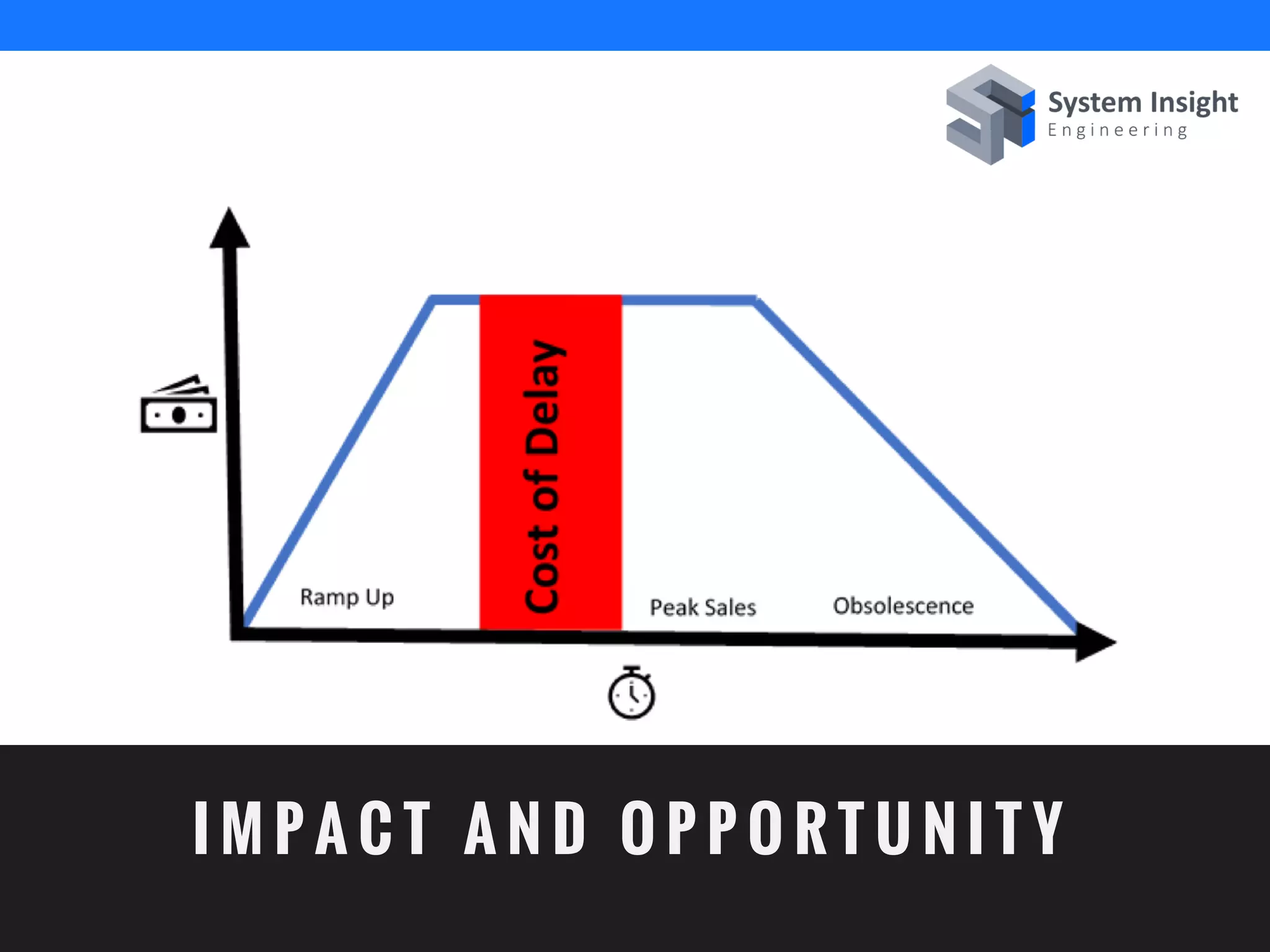
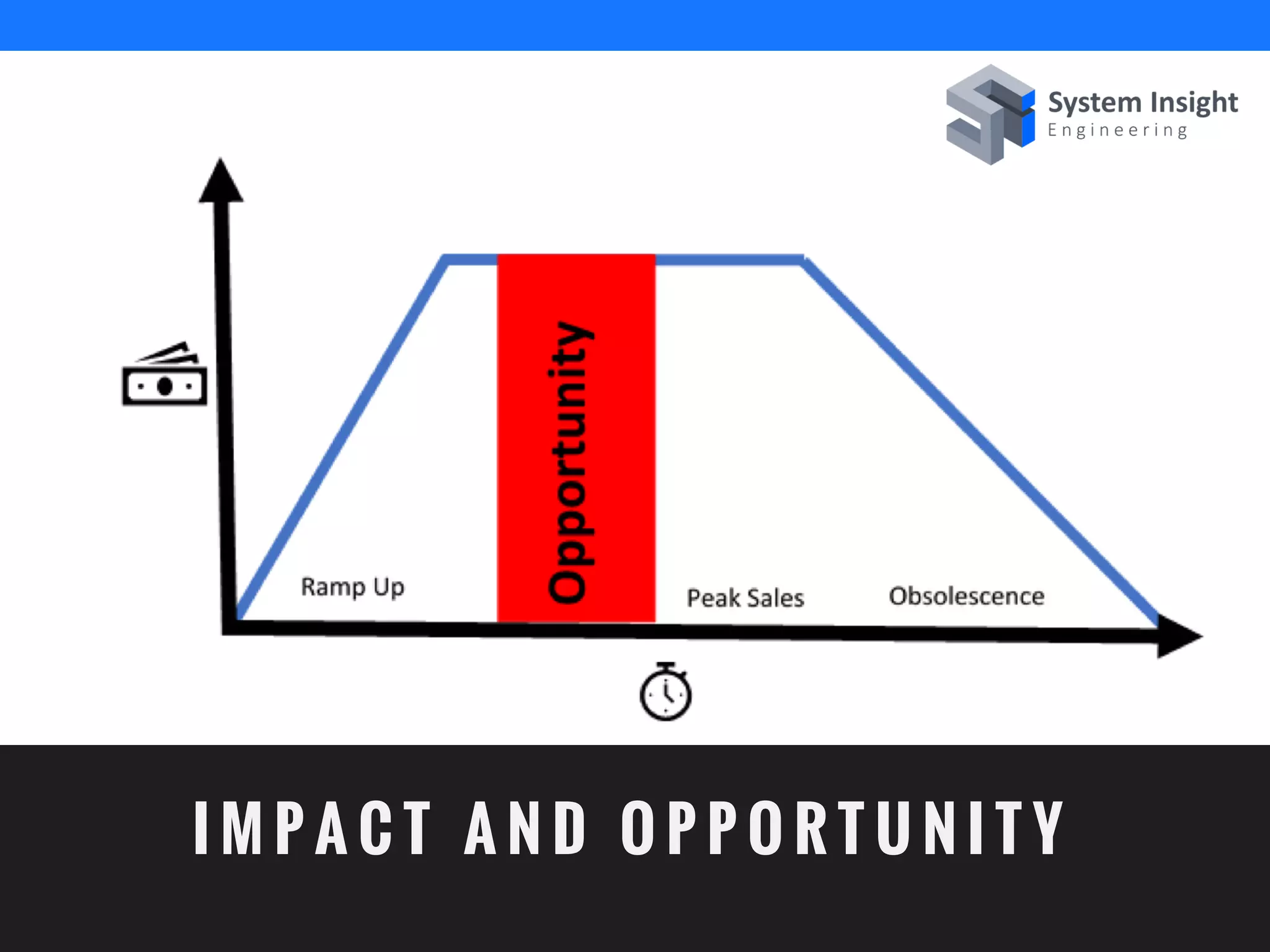
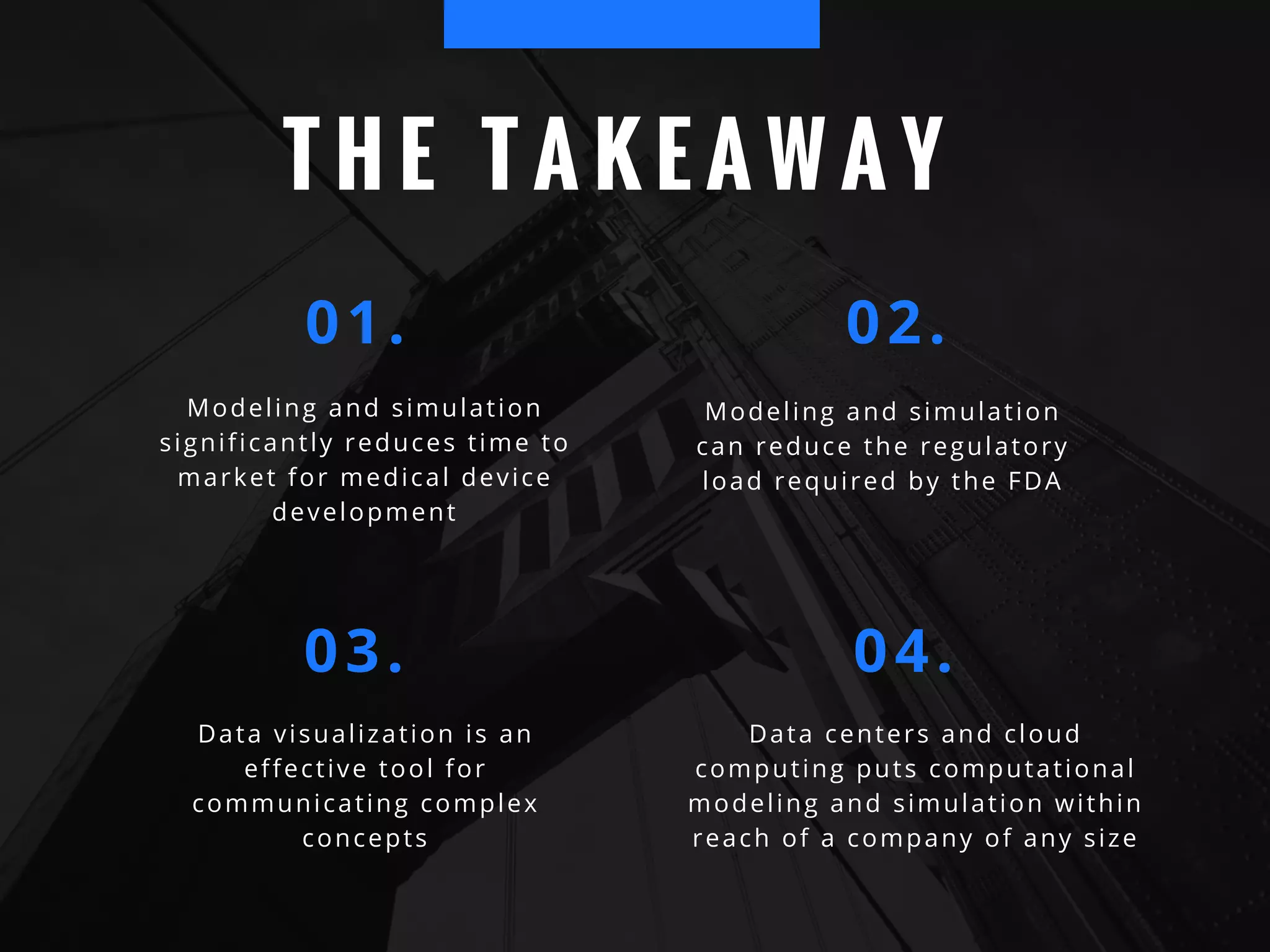
The document discusses the advantages of using computational modeling and simulation in the development of medical devices, emphasizing time and cost savings along with enhanced product quality. It highlights critical design questions, the limitations of physical prototypes, and the regulatory implications of preclinical data requirements. The presentation concludes that effective modeling and simulation, augmented by cloud computing, can streamline the regulatory process and provide effective data visualization for better communication of complex concepts.


![A L I T T L E A B O U T M E
A R L E N W A R D
15 years in Medical Device R&D [Valleylab/Covidien/Medtronic]
BS and MS degrees in Mechanical Engineering from University
of Colorado
PhD in Mechanical Engineering from Colorado State University
PHD, PE
Dissertation: “Improvements to Transurethral
Resection of Prostate (TURP) Electrosurgical
Devices through Finite Element Modeling”
Licensed PE in Colorado
17 US and 50+ Worldwide Patents](https://image.slidesharecdn.com/arlenward10xmdtx4518-180411021947/75/Computational-Modeling-and-Simulation-to-get-to-Market-Faster-3-2048.jpg)