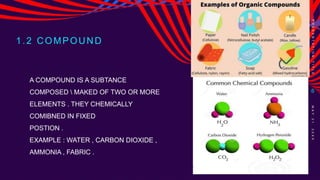
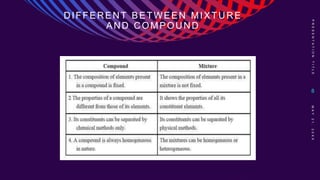
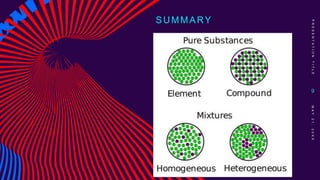
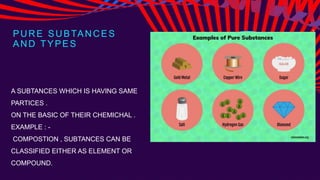
This chemistry lesson discusses pure substances and mixtures. It defines a pure substance as having the same type of particles throughout and being either an element or compound based on its chemical composition. Elements are substances that cannot be broken down further, while compounds are formed by two or more elements chemically combining in fixed ratios. Mixtures are formed when two or more pure substances are combined but do not chemically react to form a new substance. The document outlines the key properties of elements and compounds versus mixtures and provides examples of each.


![1.1 ELEMENT
ROBERT BOYLE WAS THE FIRST
SCIENTIST TO USE THR TERM ELEMENT .
ANTOINE LAURENT LAVOISER [1743 - 1794]
, A FRENCH CHEMIST WAS THE FRIST TO
ESTABISH AN EXPERIMENTALLY USEFUL
DEFINITION OF AN ELEMENT
P
R
E
S
E
N
T
A
T
I
O
N
T
I
T
L
E
4
M
A
Y
2
1
,
2
0
X
X](https://image.slidesharecdn.com/chemistryls2pptina-220824123817-b408ffb2/85/Class-9-Chemistry-LS-2-PPT-INA-pptx-4-320.jpg)