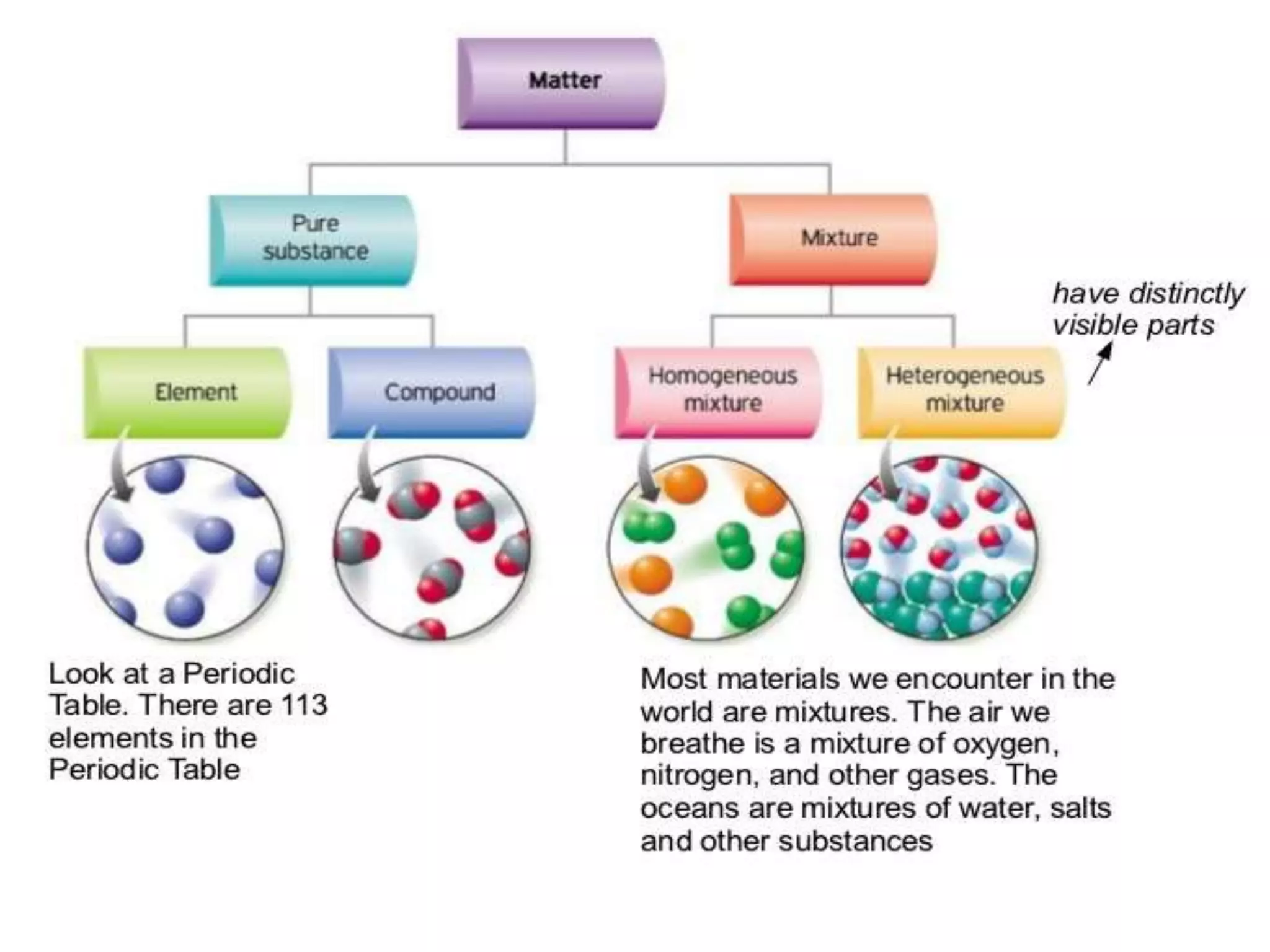
An atom is the basic unit of an element. Elements are pure substances made of only one type of atom.
A molecule is formed when two or more atoms of one or more elements are chemically bonded together. Compounds are pure substances made of molecules containing two or more elements chemically bonded together in a fixed ratio.
So in summary:
- An atom is to an element as the basic unit is to the pure substance made of that unit.
- An atom is to a molecule as the individual unit is to the combined structure formed from two or more bonded units.
- An atom is to a compound molecule as the individual unit is to the combined structure formed from two or more different bonded units that make