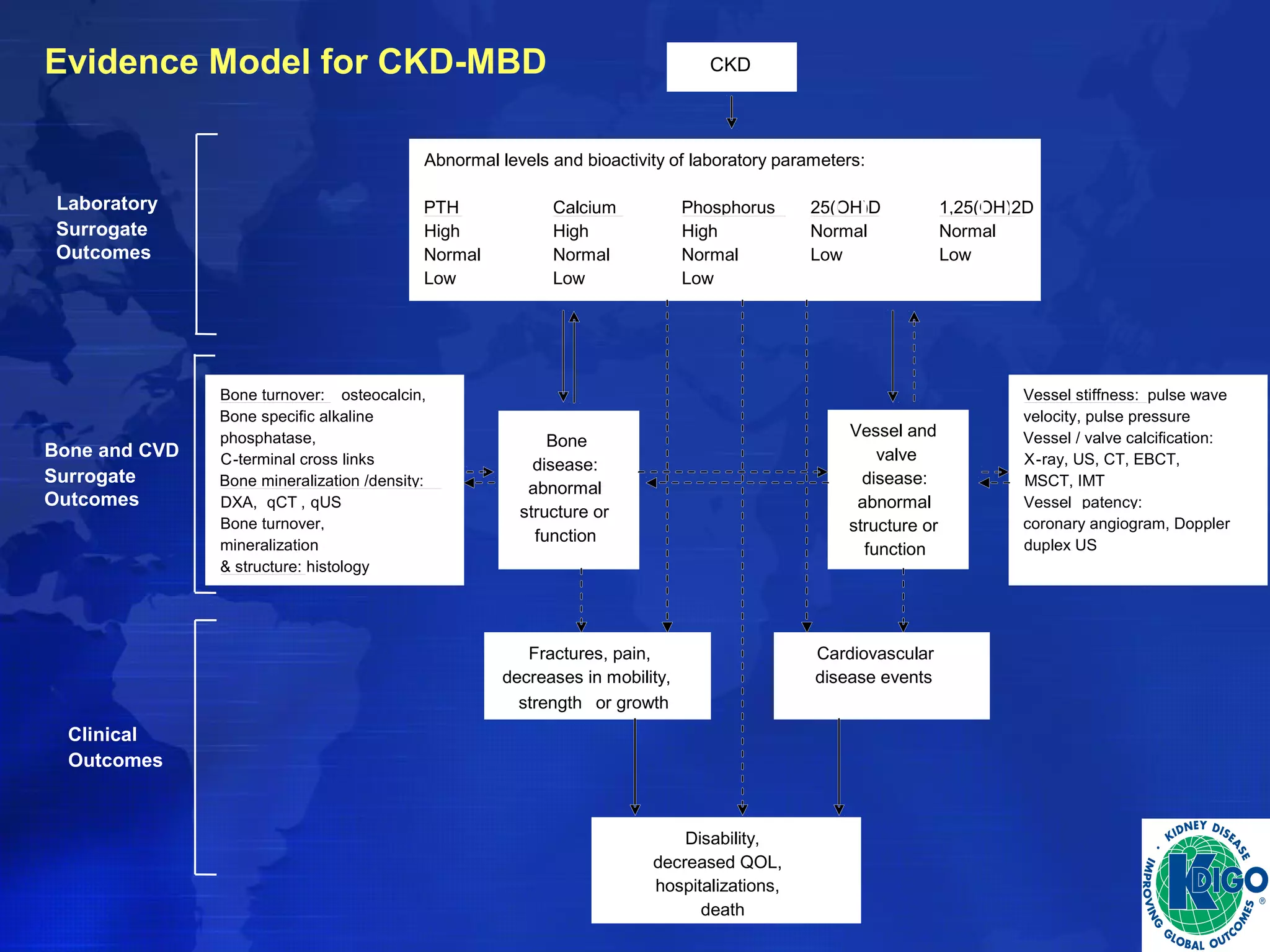
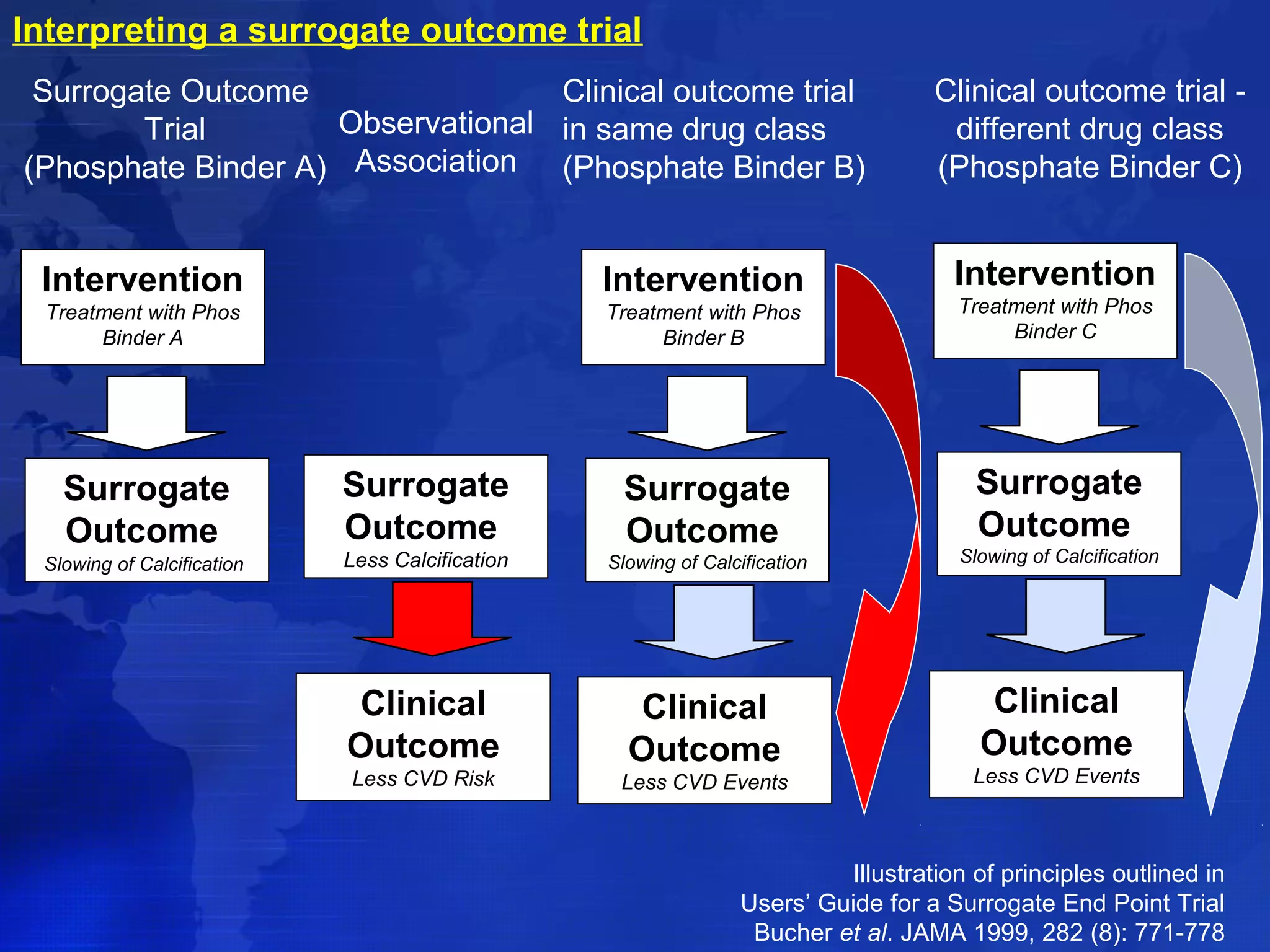
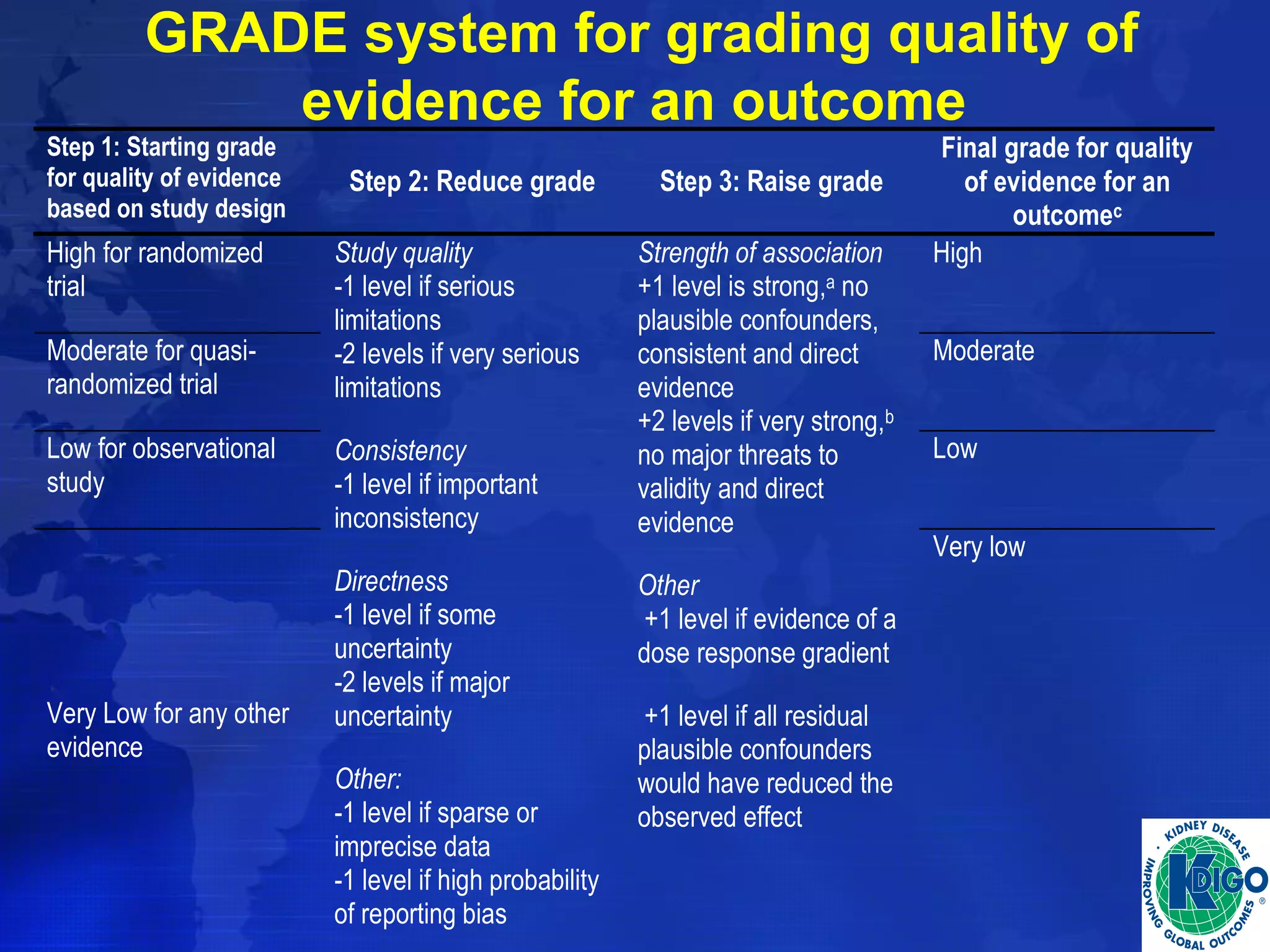
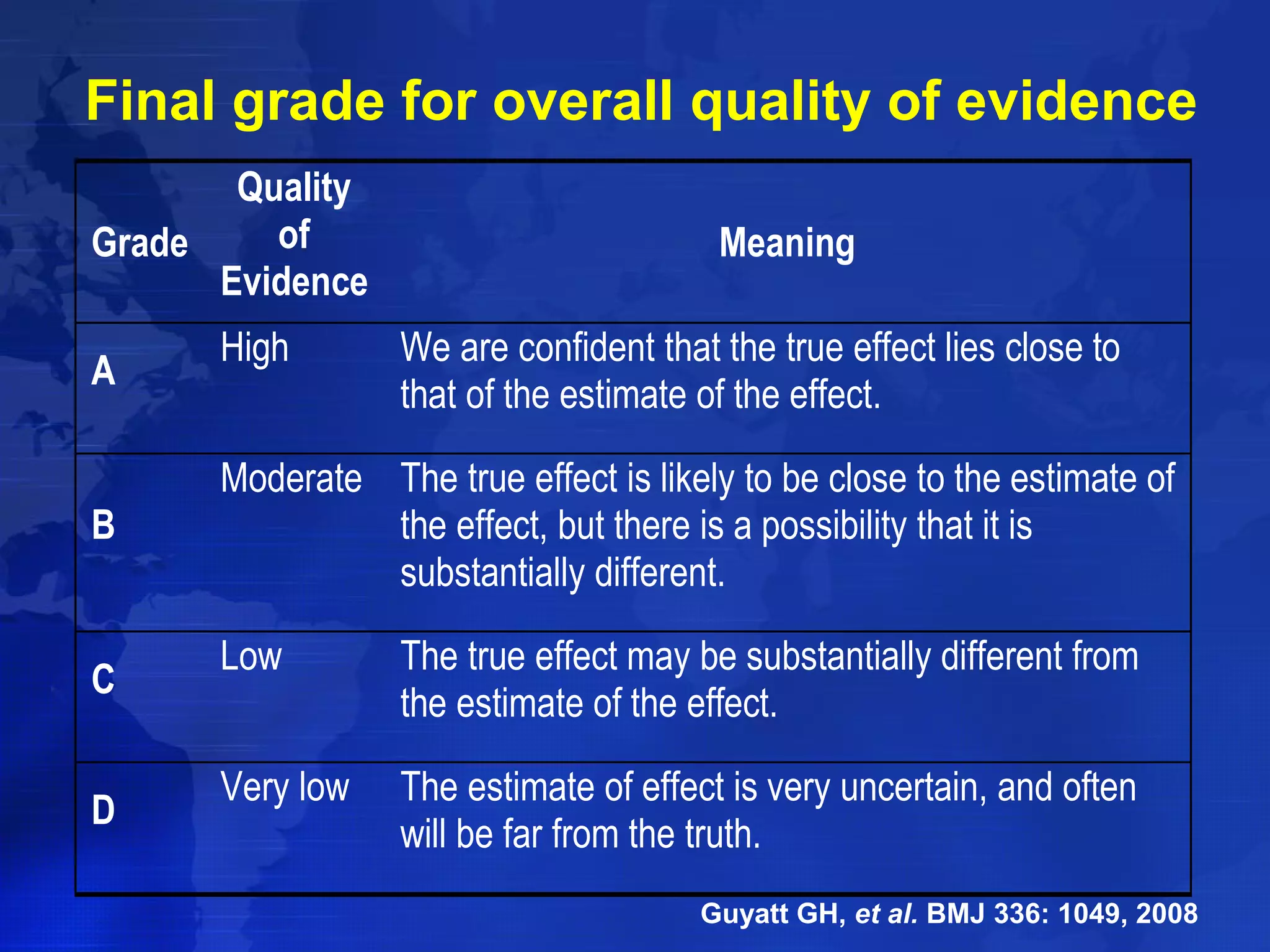
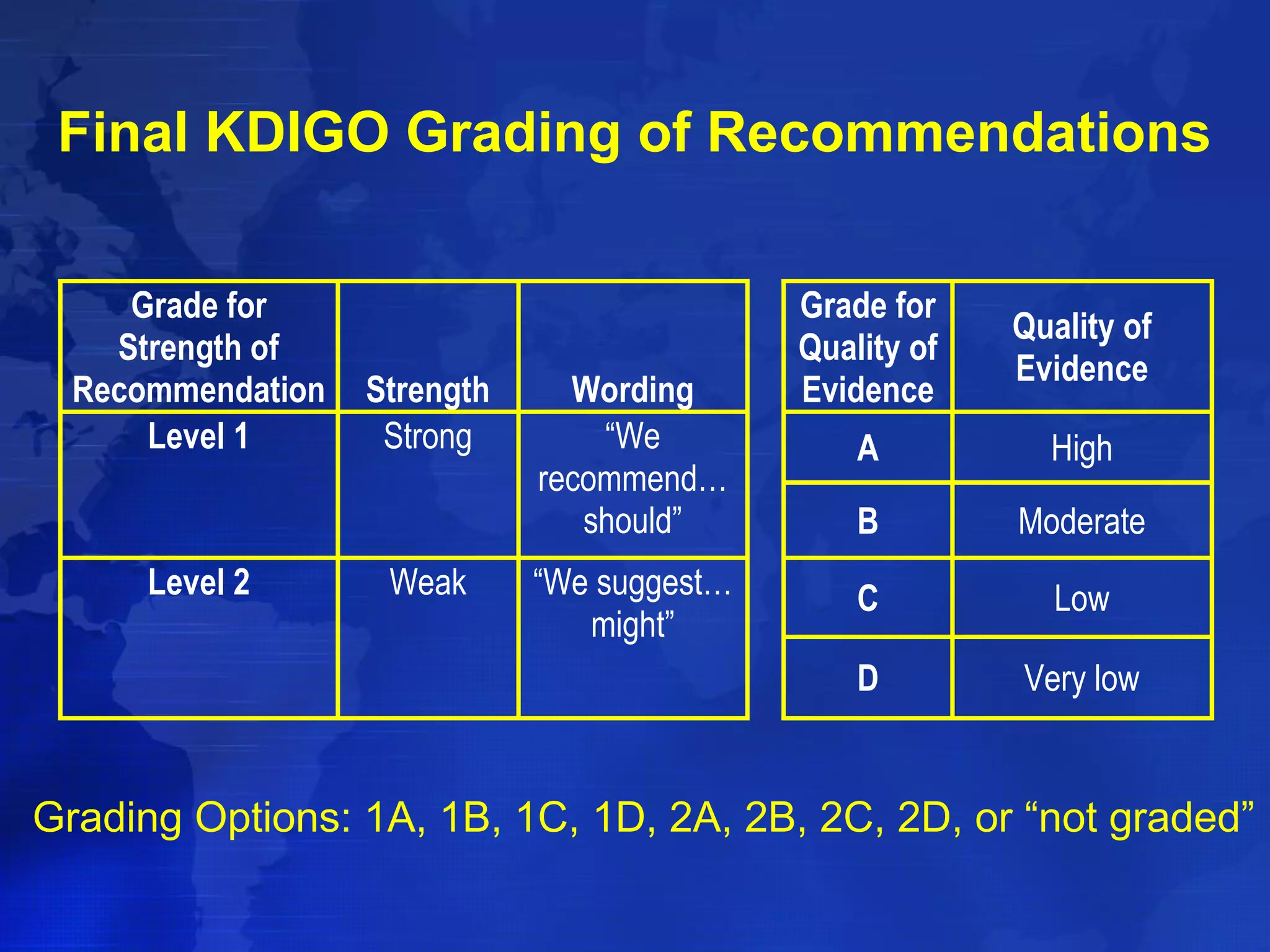
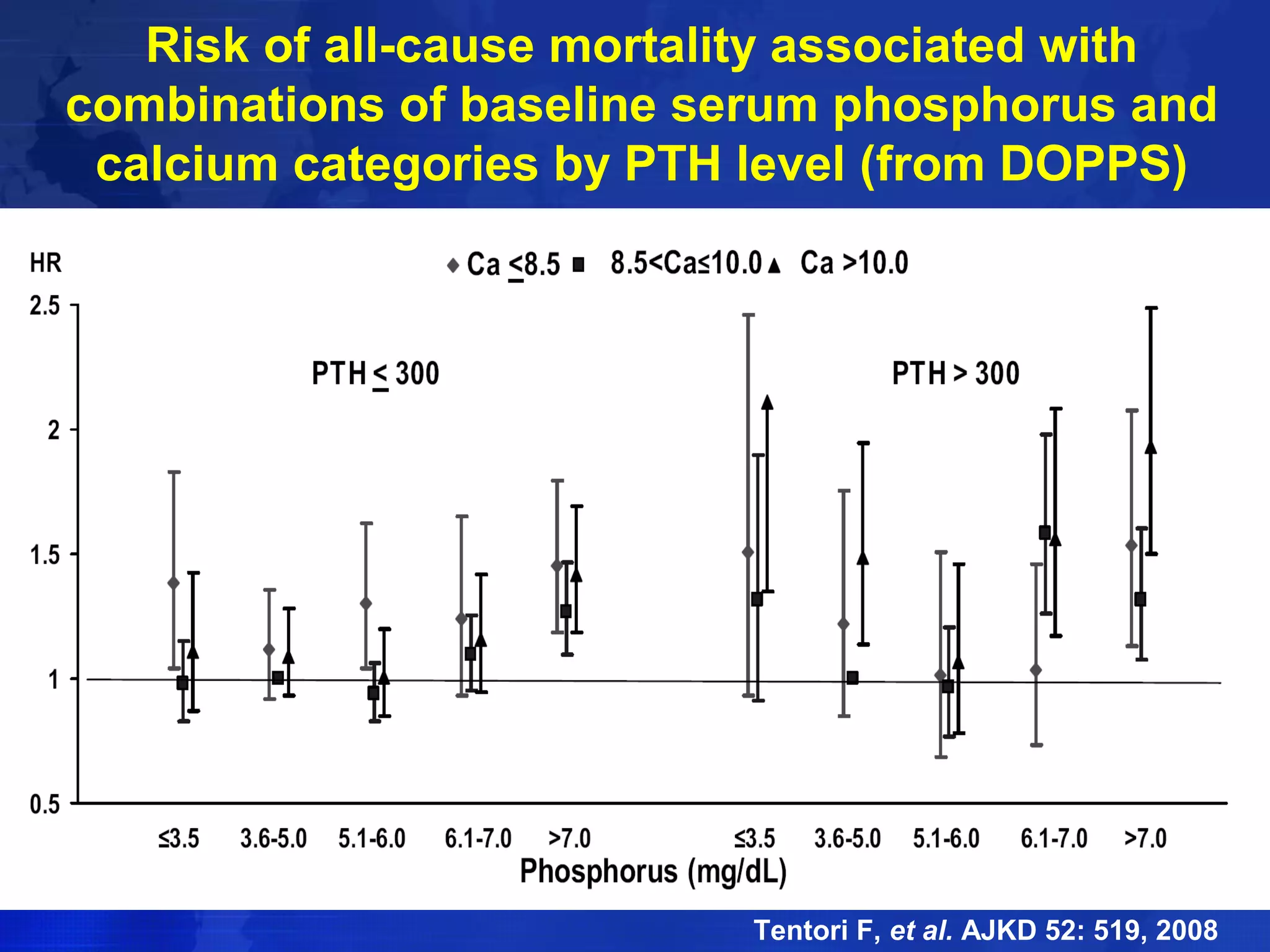
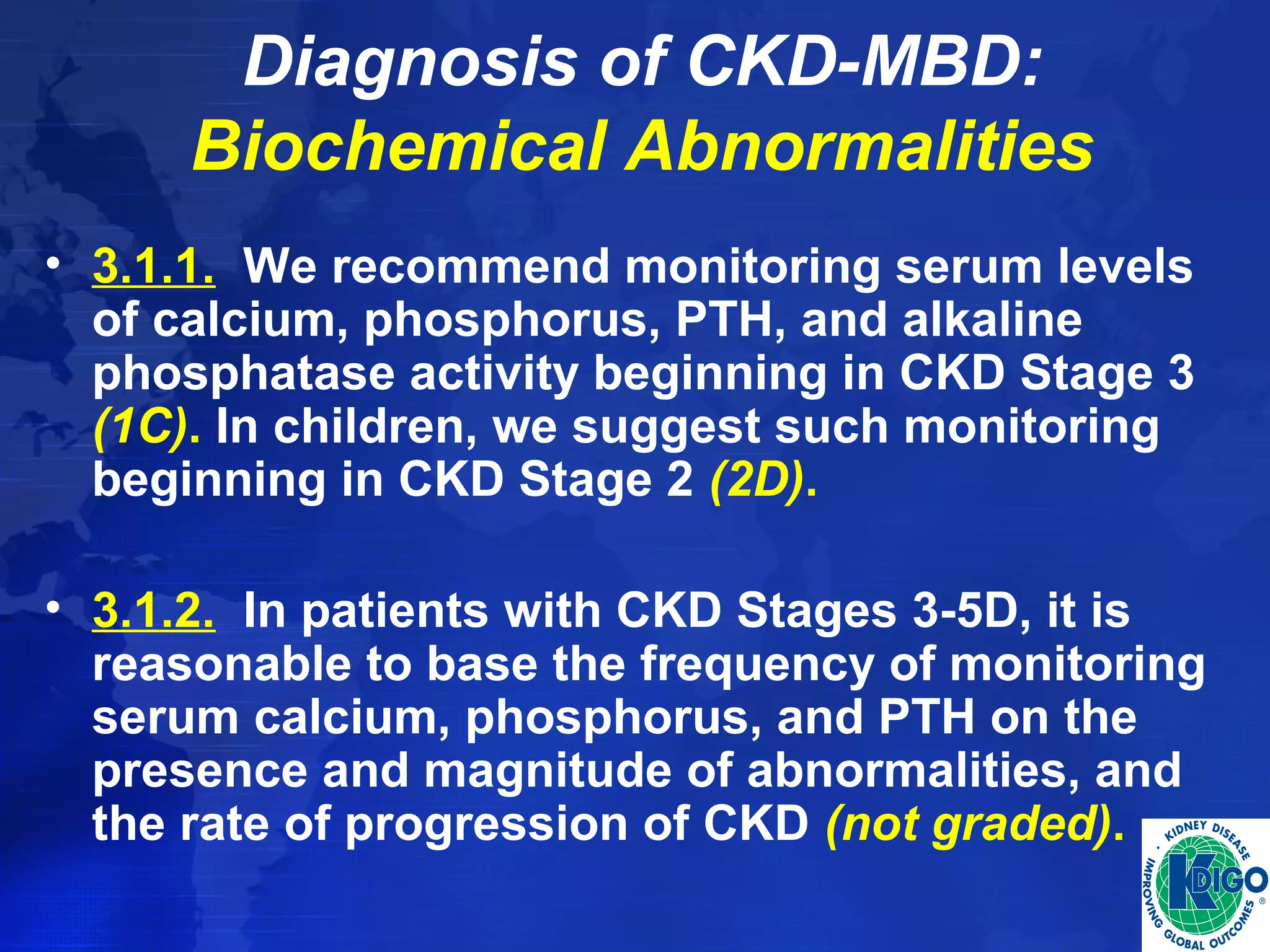
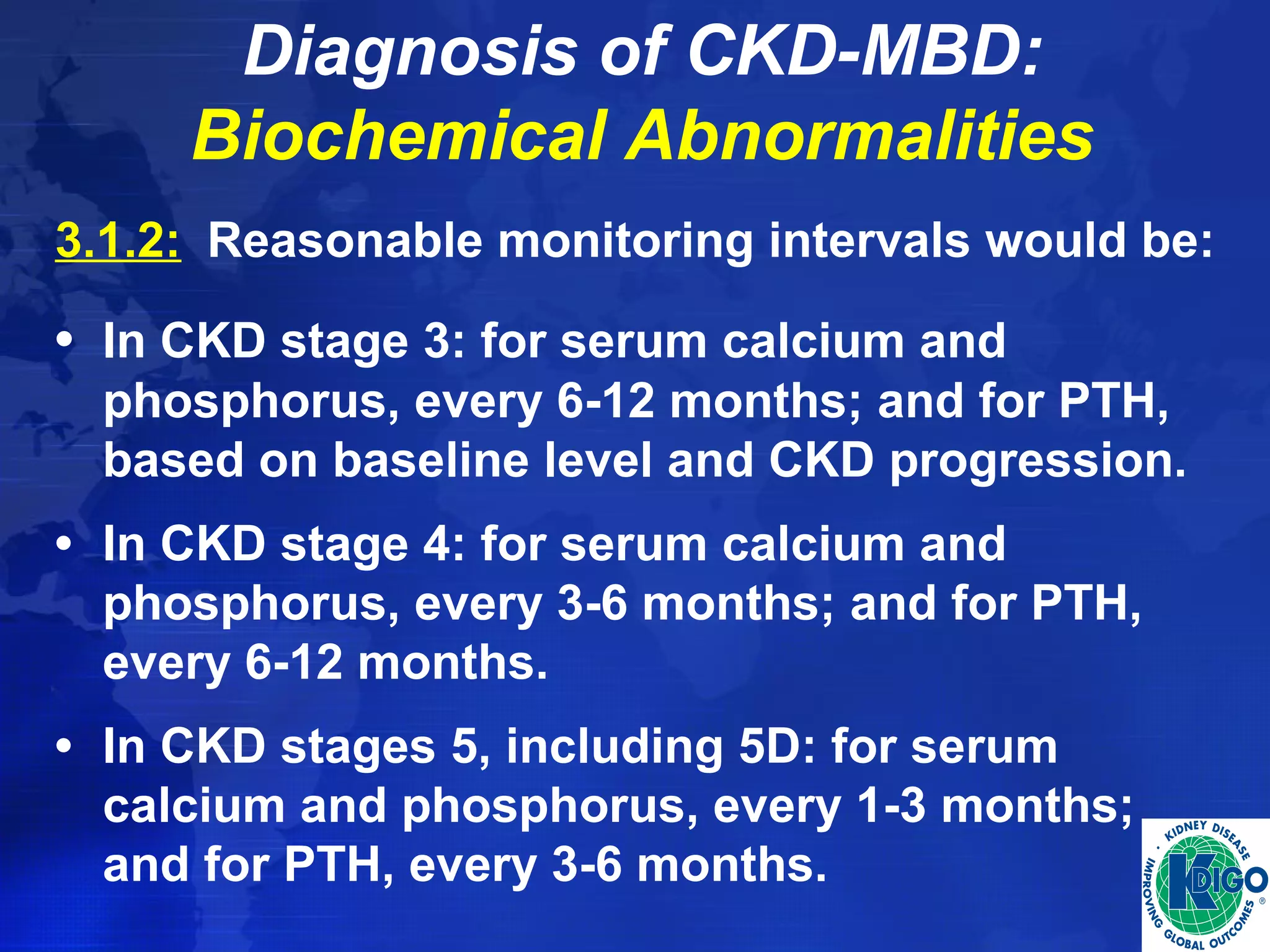
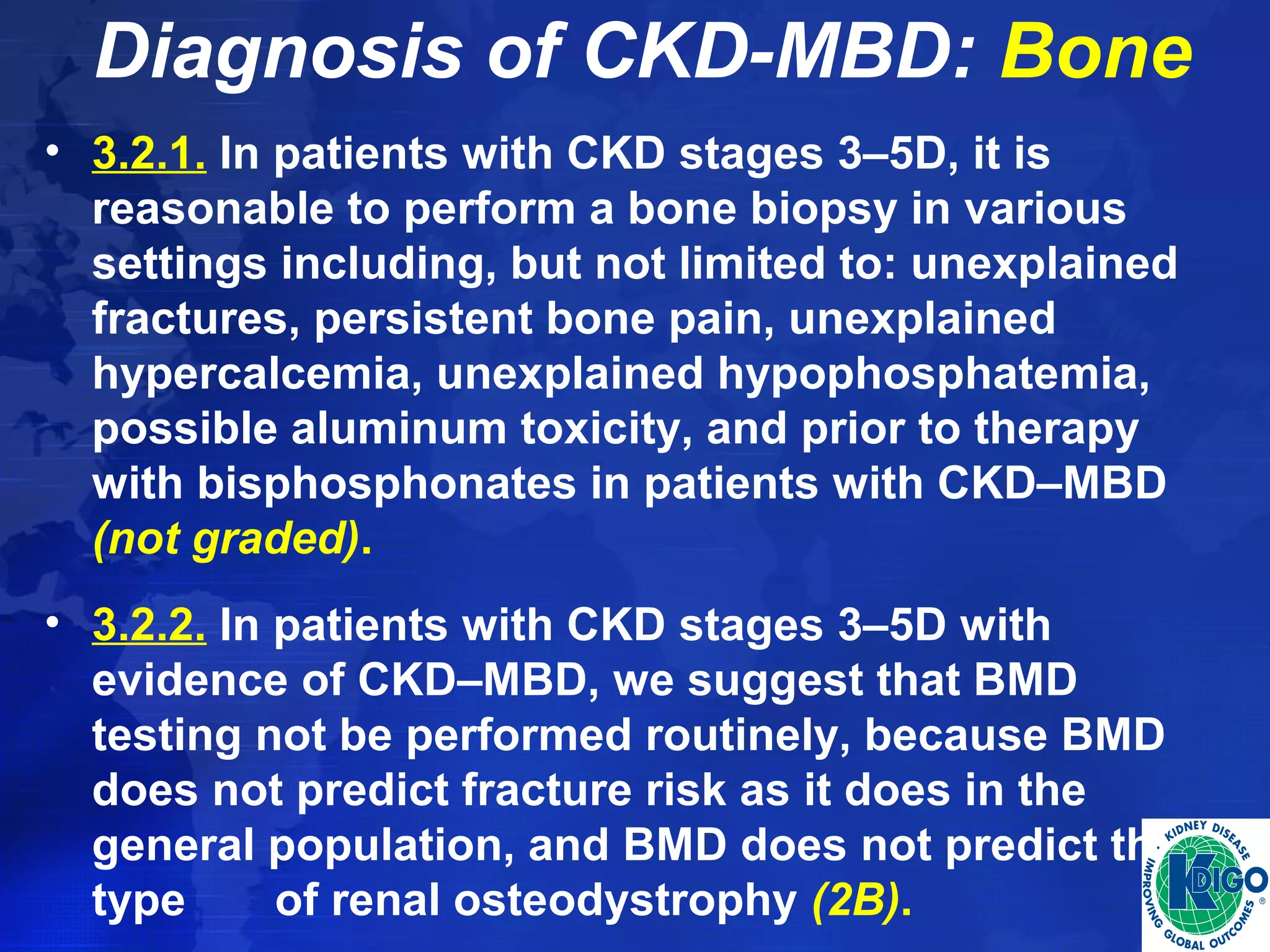
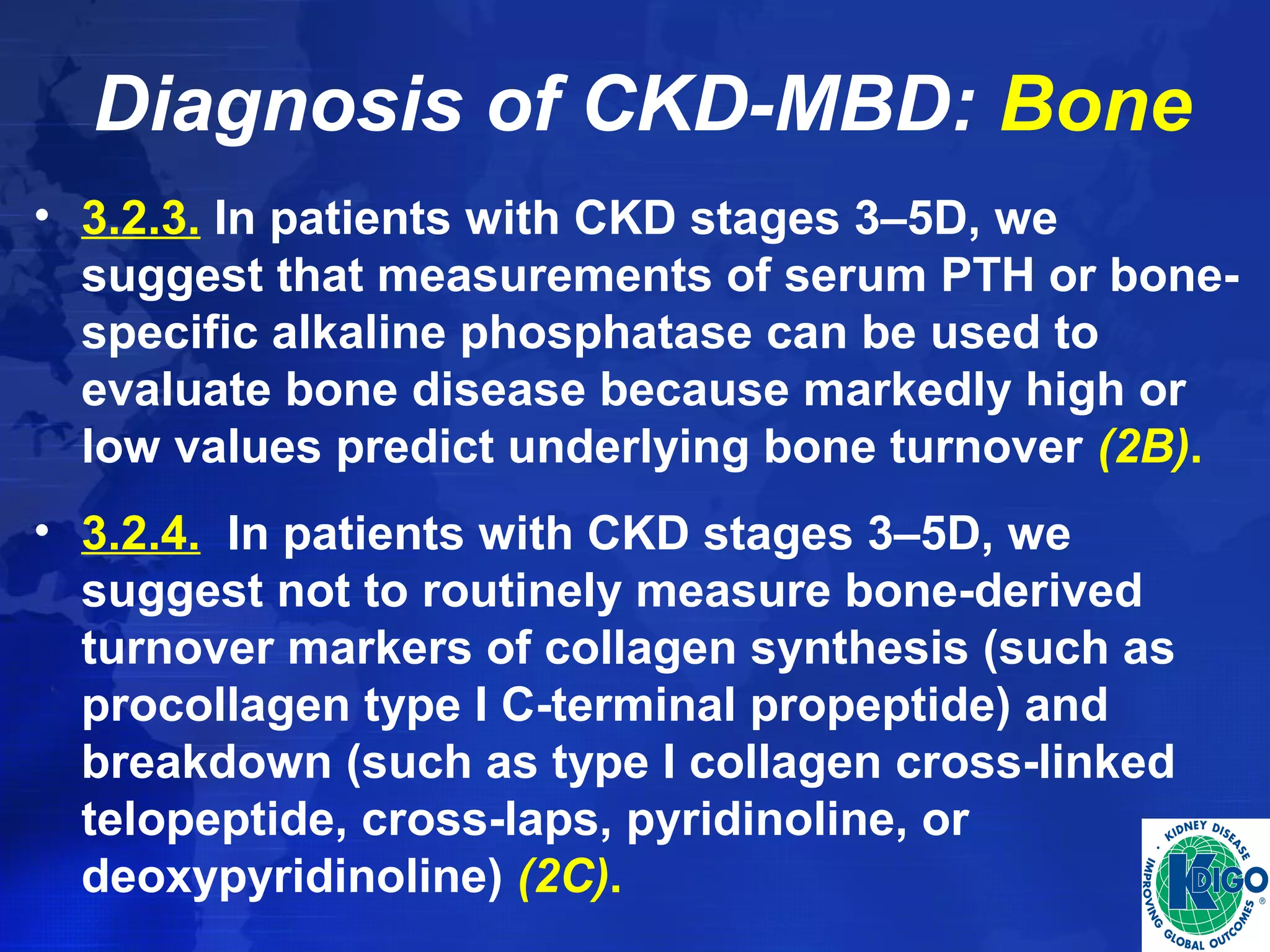
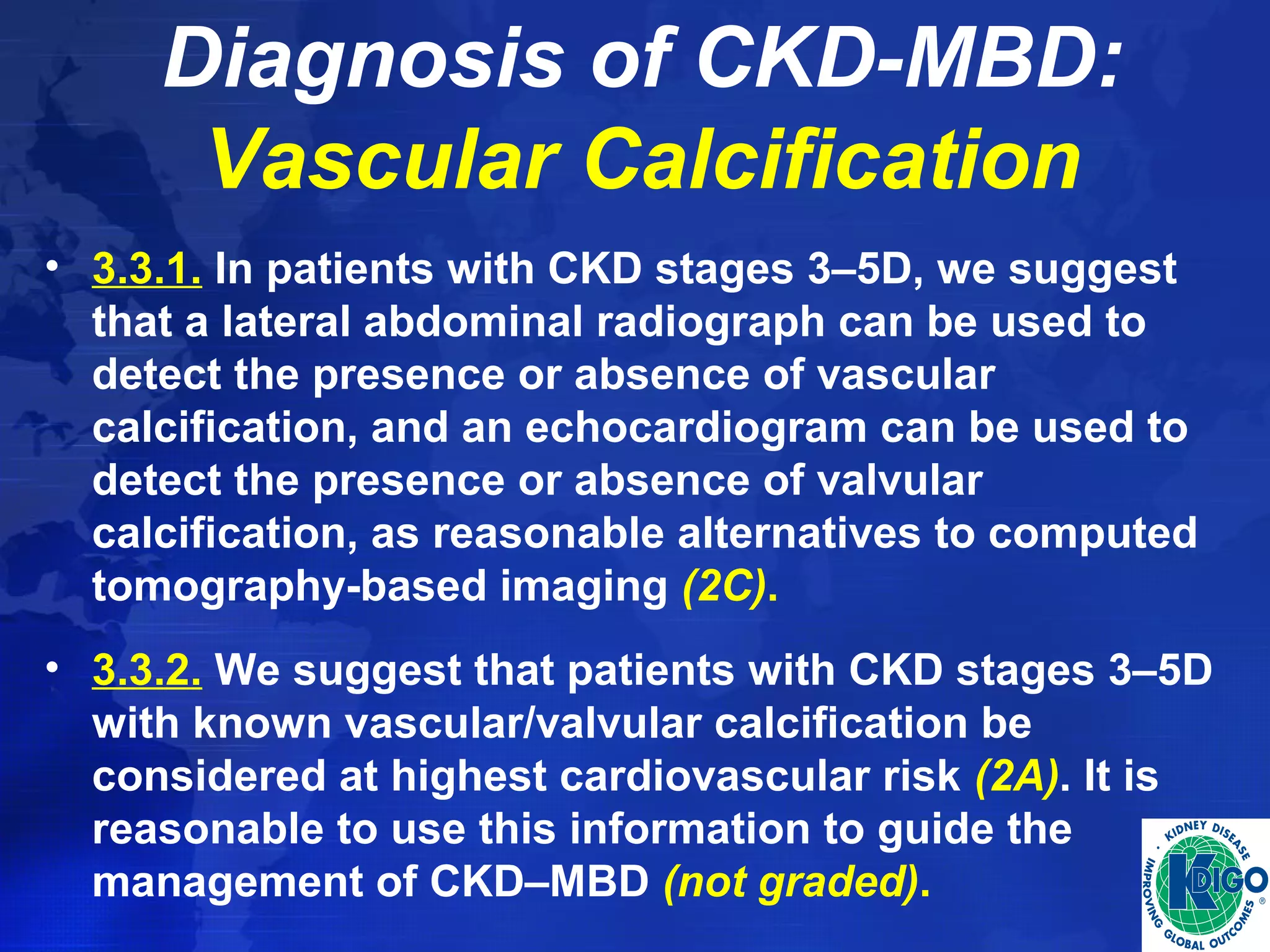
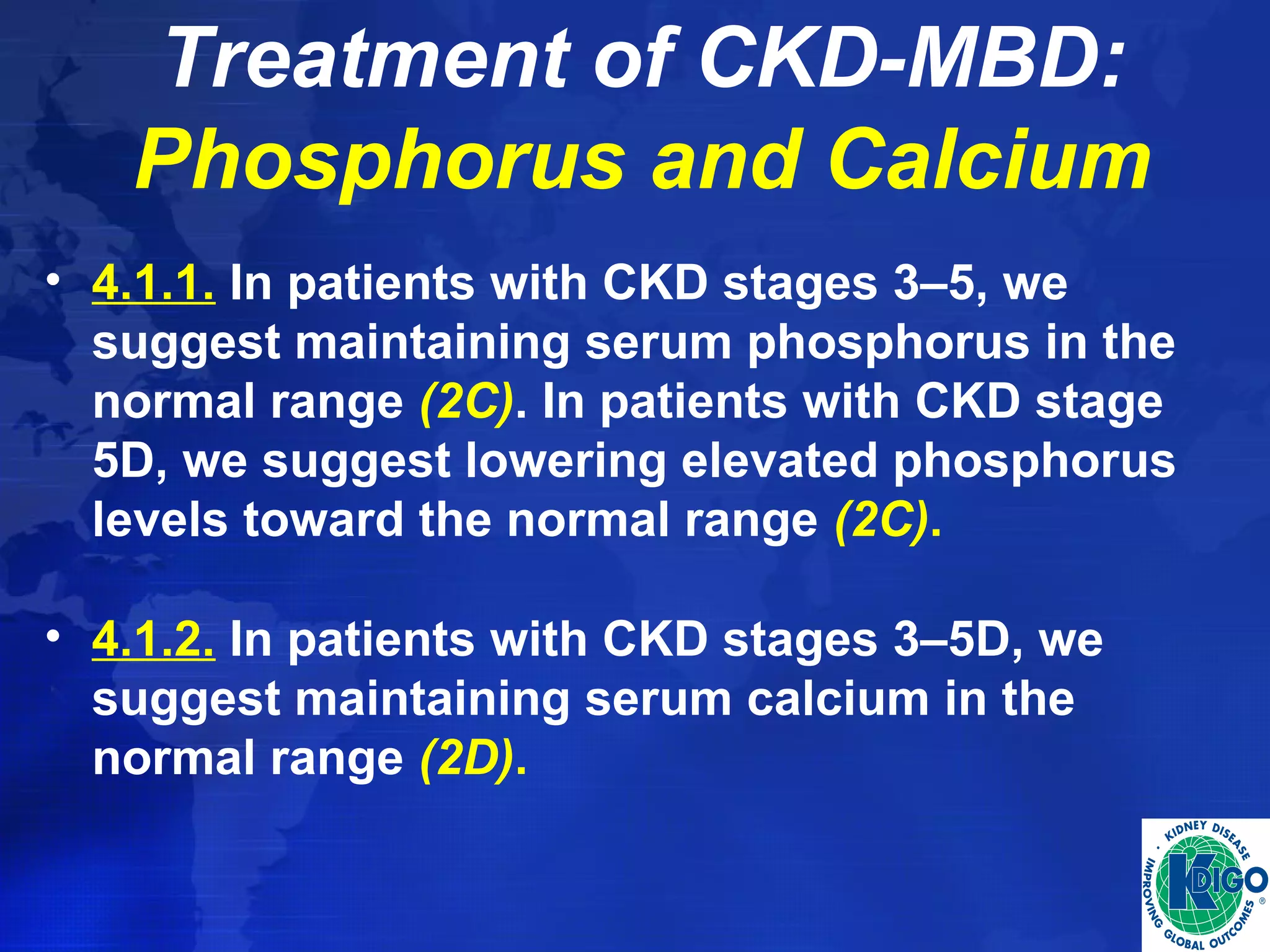
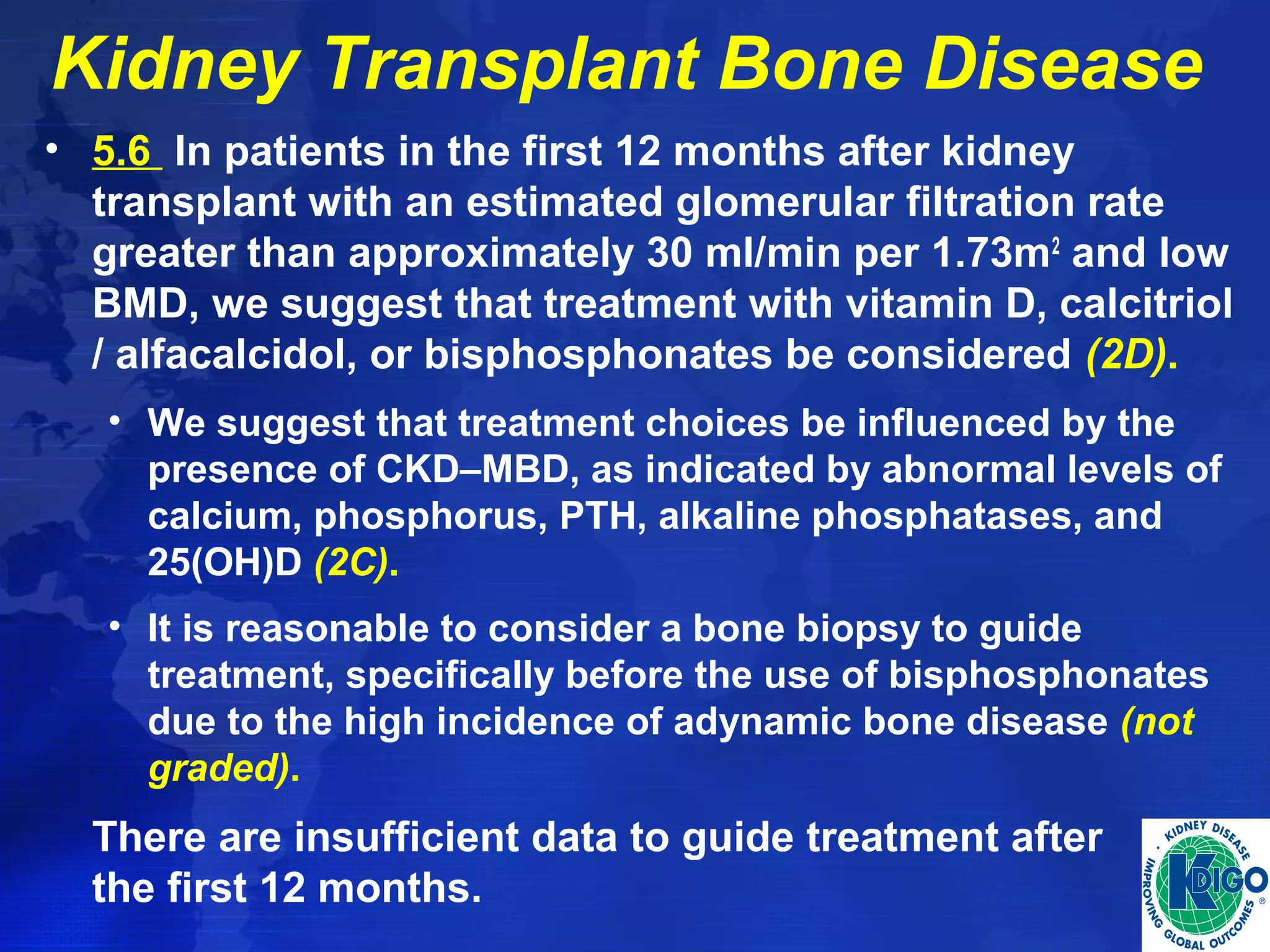
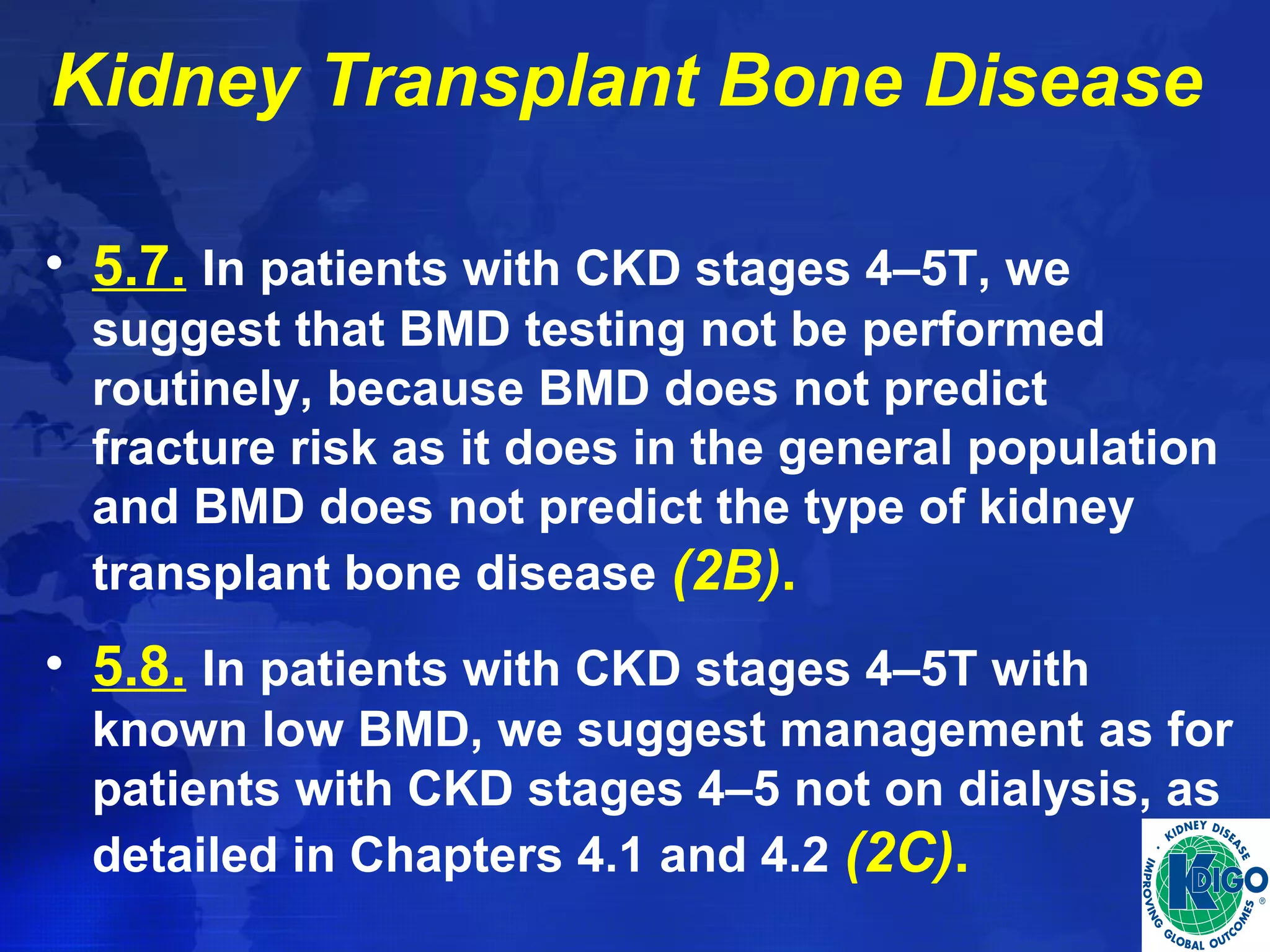
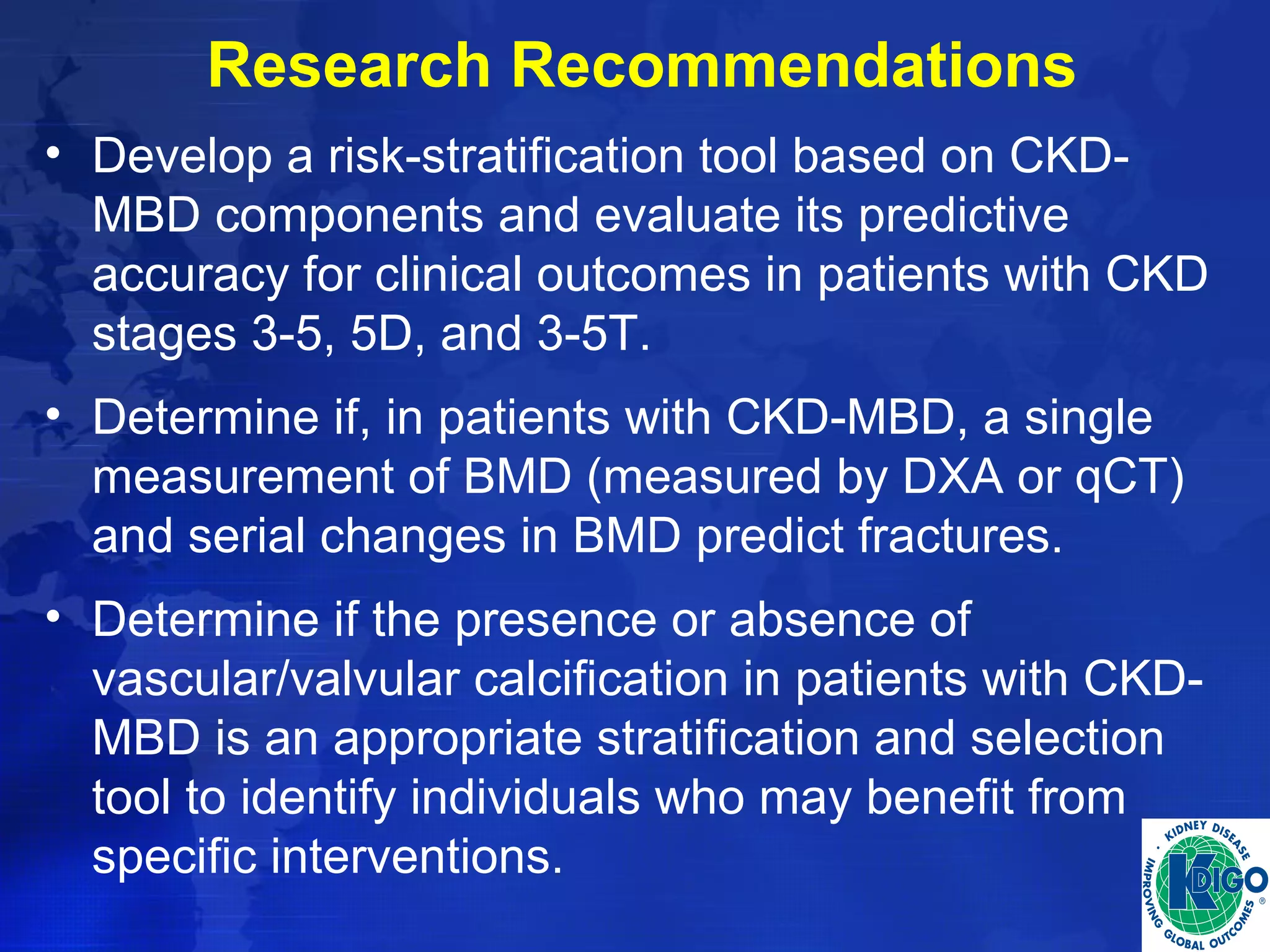
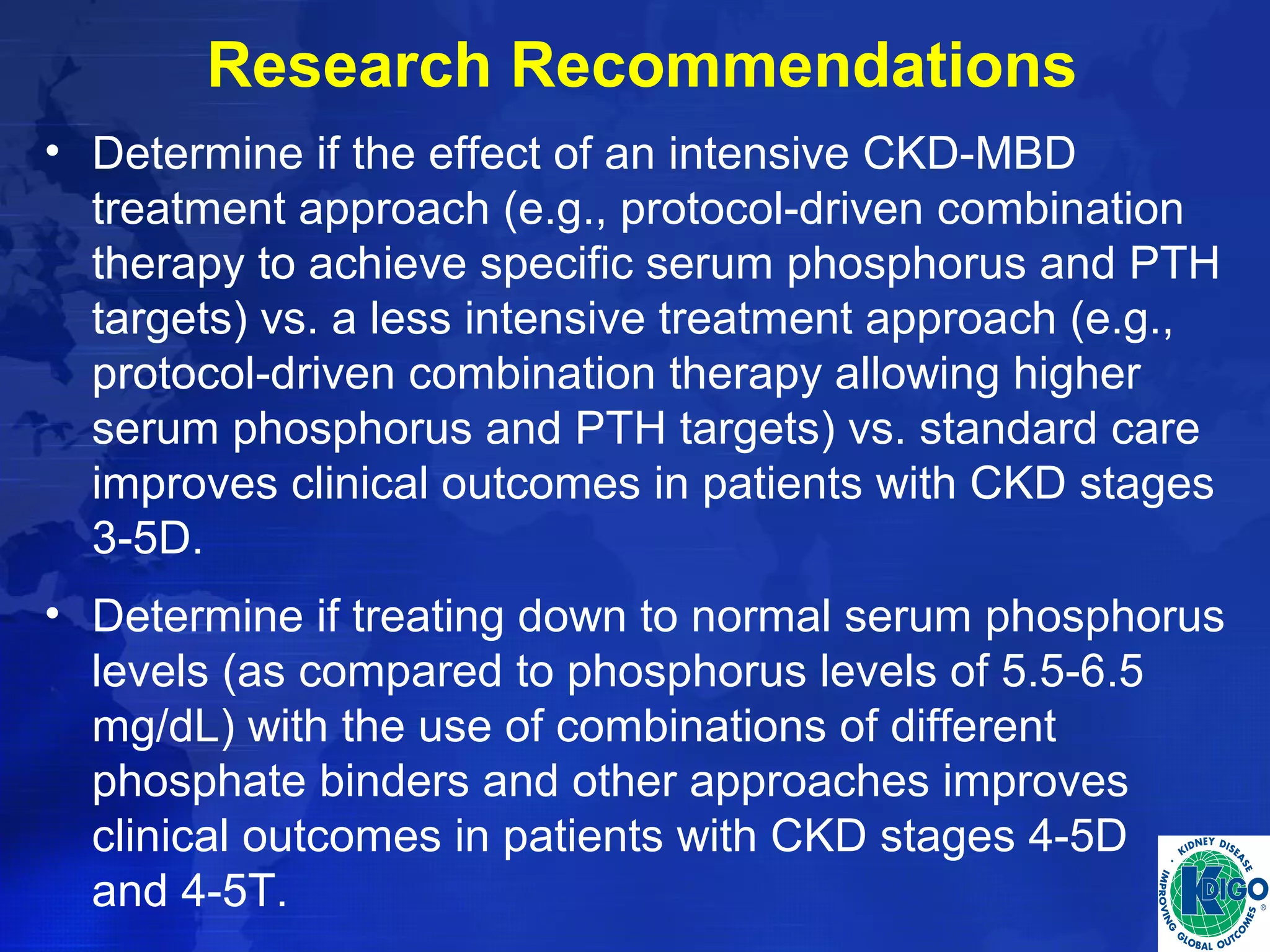
This document provides guidelines for the diagnosis, evaluation, prevention and treatment of chronic kidney disease - mineral and bone disorder (CKD-MBD). It was developed by an international work group convened by Kidney Disease: Improving Global Outcomes (KDIGO). The guidelines include recommendations for monitoring laboratory parameters like calcium, phosphorus and PTH levels at different stages of CKD. They also provide guidance on evaluating and managing bone disease in CKD patients, including suggestions on using bone biopsies and bone mineral density tests. The guidelines are meant to help standardize and improve the care of CKD-MBD worldwide.



























![Research Recommendations
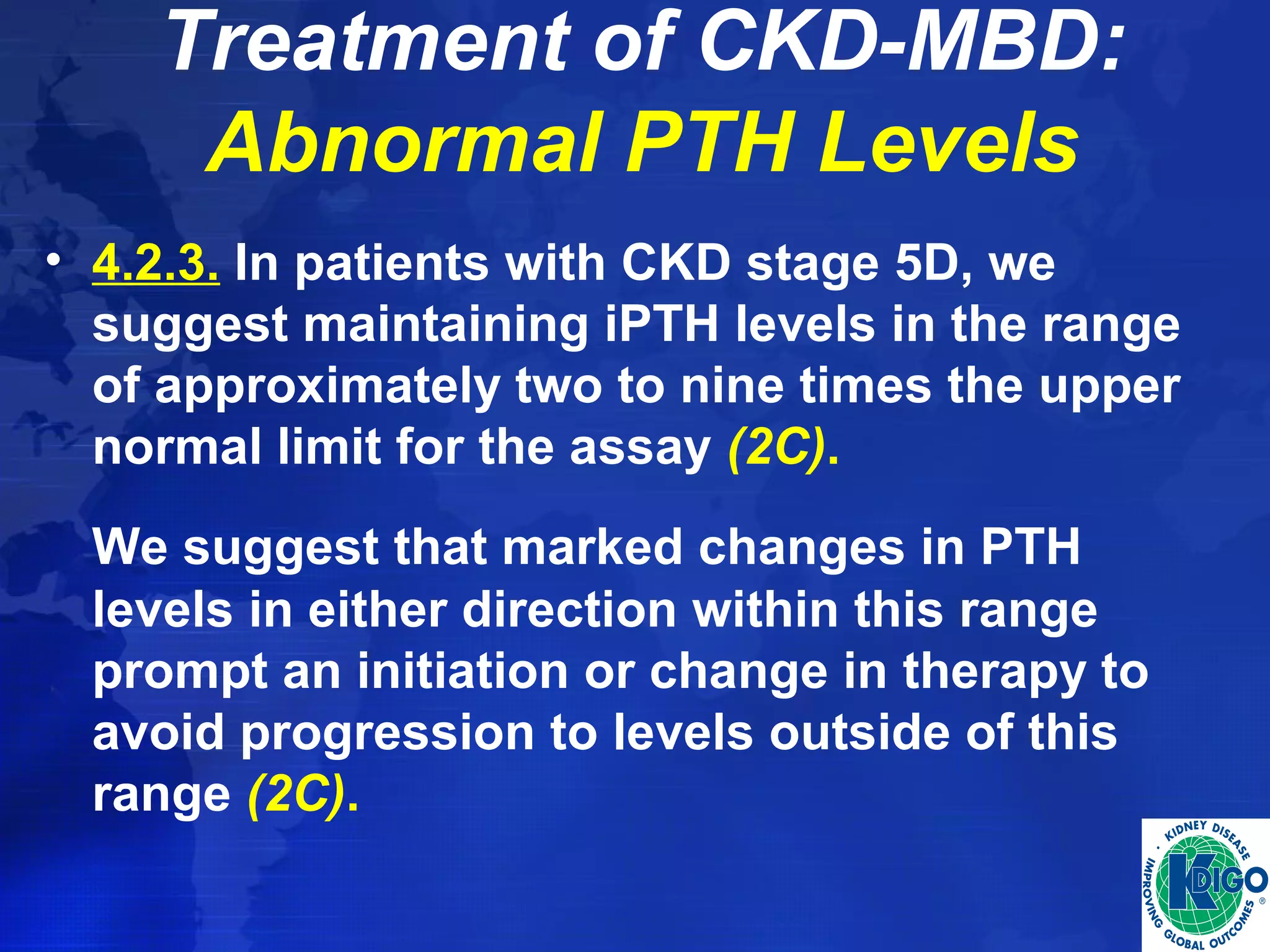
• Determine if treatment to a lower vs. a higher serum
PTH target improves or worsens clinical outcomes
in patients with CKD stages 3-5, CKD stage 5D, and
CKD stages 3-5T.
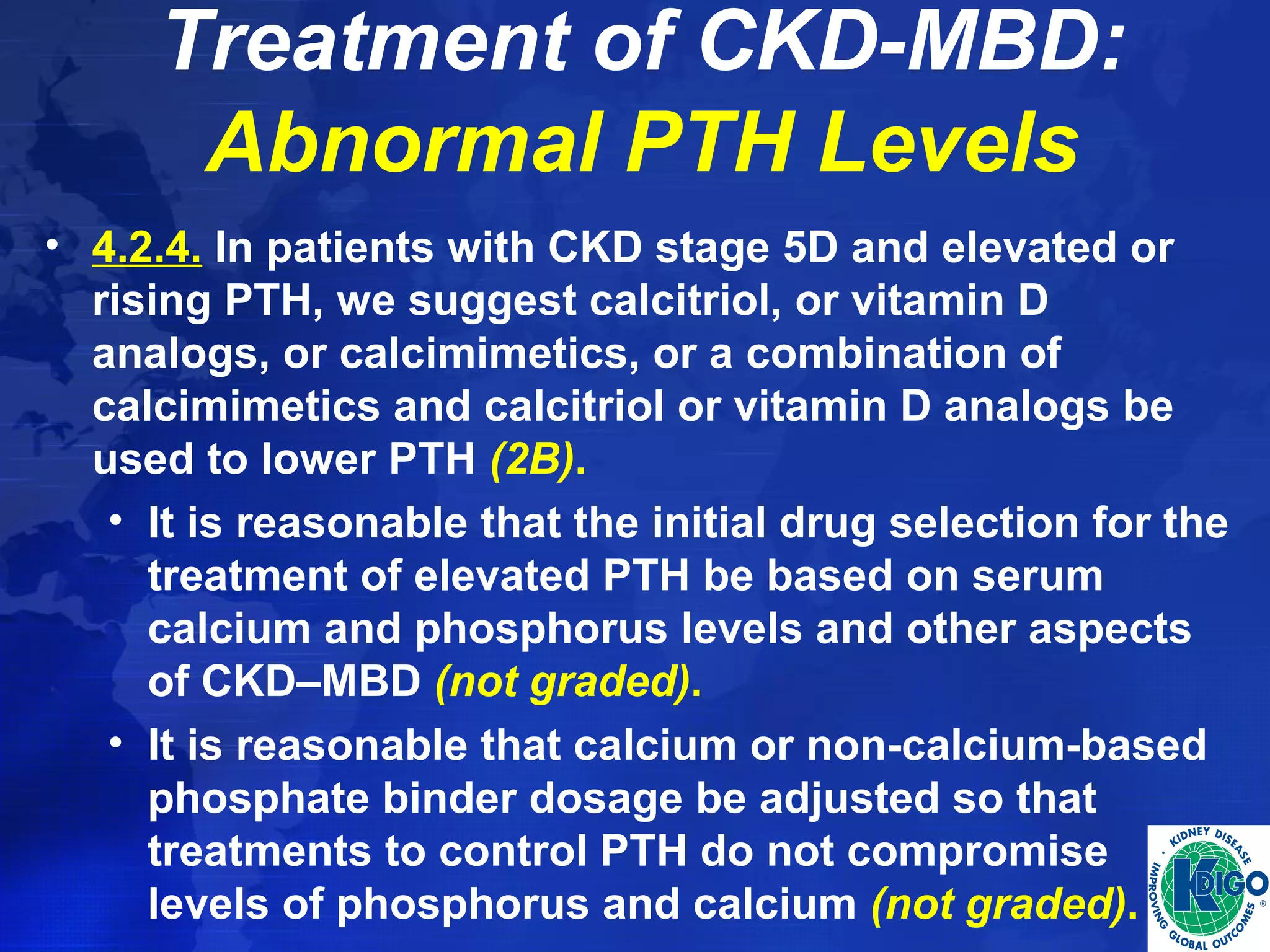
• Determine if treatment with vitamin D (ergocalciferol
or cholecalciferol) or calcidiol [25(OH)D], compared
to calcitriol or vitamin D analogs, improves clinical
outcomes in patients with CKD stages 3-5, CKD
stage 5D, and CKD stages 1-5T.
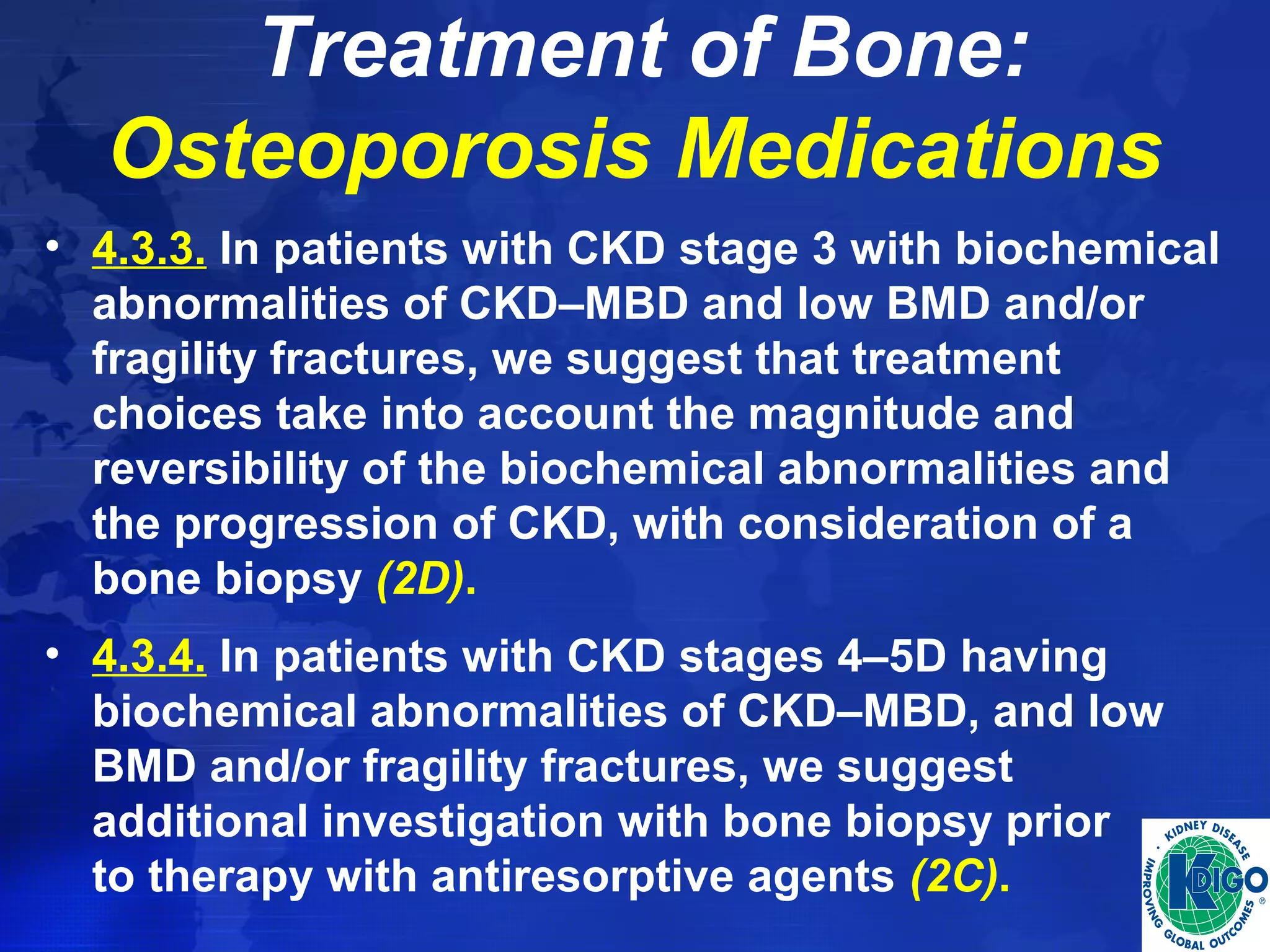
• Determine which phosphate binders and other
serum phosphorus–lowering treatments are able to
improve survival in patients with CKD
stages 3-5D and CKD stages 3-5T.](https://image.slidesharecdn.com/ckd-mbdguideline-141027014239-conversion-gate02/75/Ckd-mbd-guideline-63-2048.jpg)