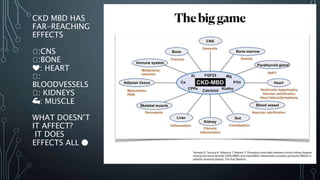
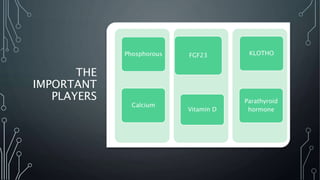
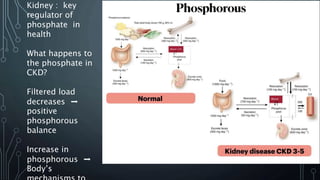
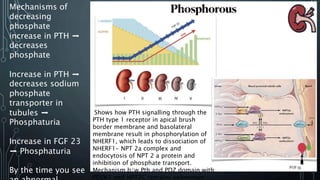
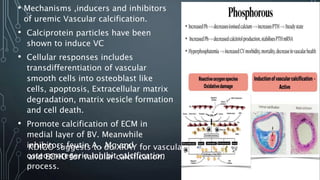
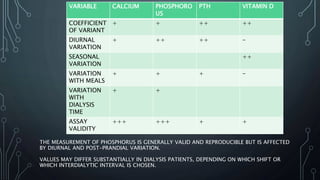
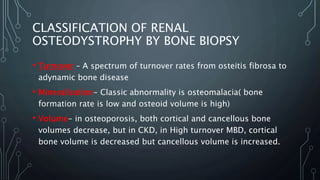
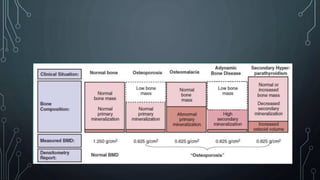
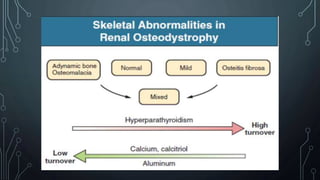
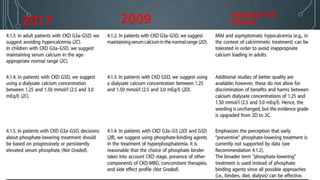
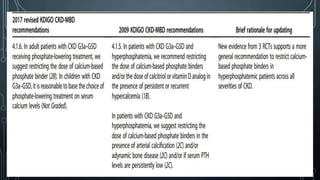
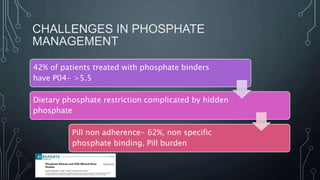
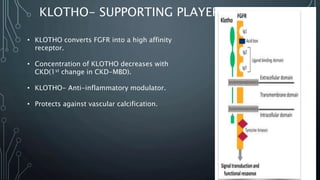
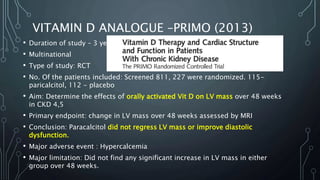
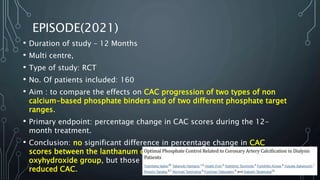
The document summarizes guidelines from KDIGO on chronic kidney disease-mineral and bone disorder (CKD-MBD). It discusses the components and basics of CKD-MBD, including the key players involved like phosphorus, calcium, FGF23, and vitamin D. It also summarizes sections from the guidelines on diagnosis of CKD-MBD through biochemical abnormalities, bone abnormalities, and vascular calcification. Management guidelines are summarized for targeting high serum phosphate and PTH levels. Several trials evaluating therapies for CKD-MBD like vitamin D analogues, phosphate binders, and calcimimetics are also briefly summarized.