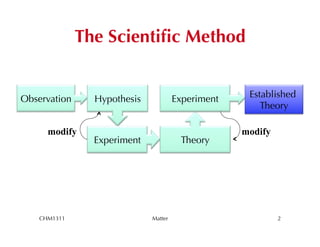
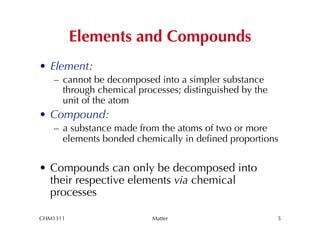
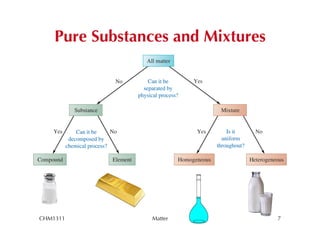
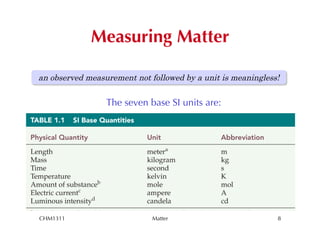
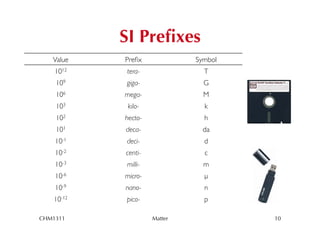
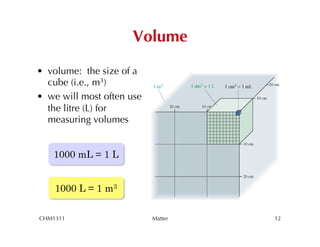
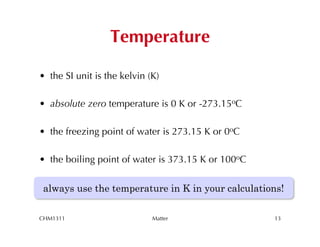
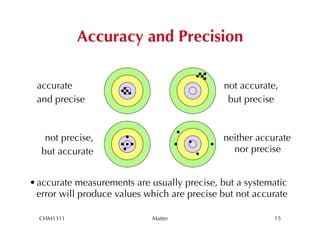
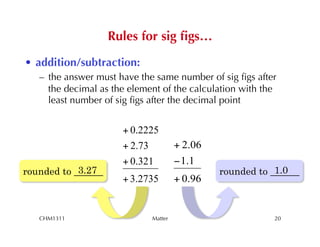
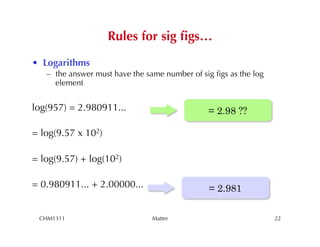
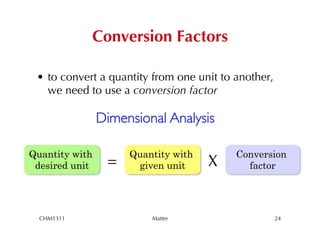
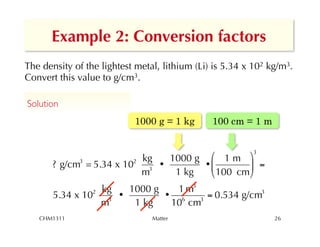
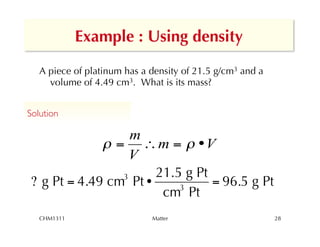
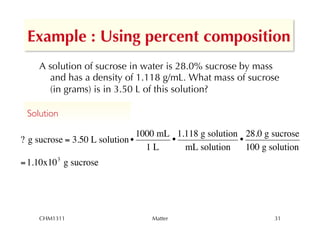
This chapter introduces fundamental concepts of matter including its properties and measurement. It discusses the scientific method and defines key terms like element, compound, mixture, and properties. It also covers units of measurement, accuracy and precision, significant figures, density, and using conversion factors to interconvert between units. The chapter establishes a foundation for quantitative work in chemistry.