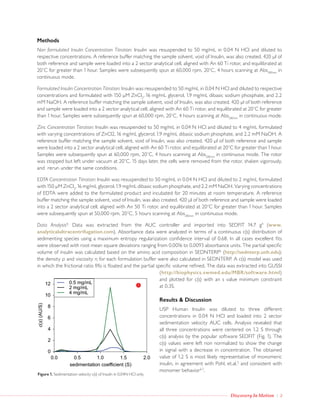
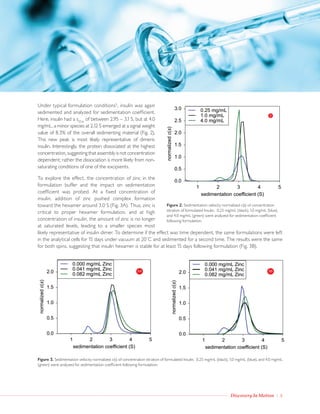
Analytical ultracentrifugation was used to characterize the higher-order structure of insulin under typical formulation conditions. It was found that zinc is critical for proper hexamer formation, while the chelating agent EDTA dissociated the hexamer into monomer and dimer species by removing zinc in a concentration-dependent manner. The zinc hexamer was stable over 15 days, while increasing insulin concentration above the saturating level of excipients led to dissociation into dimer. Analytical ultracentrifugation provides a means to study non-covalent oligomeric states of biopharmaceuticals like insulin under formulation conditions.