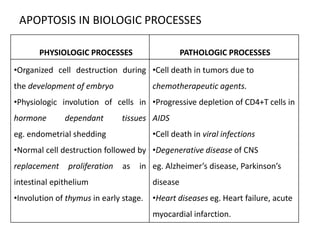
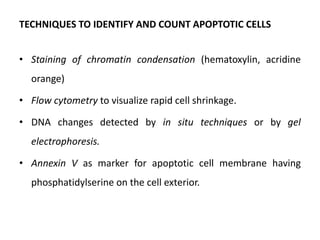
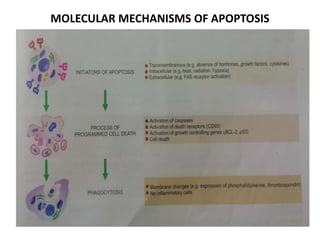
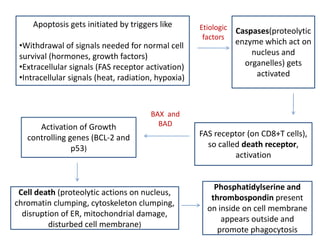
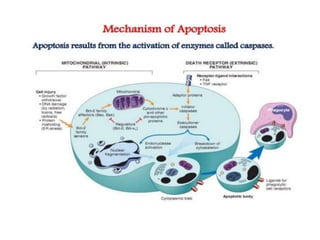
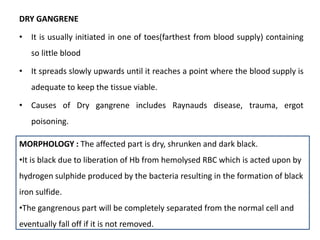
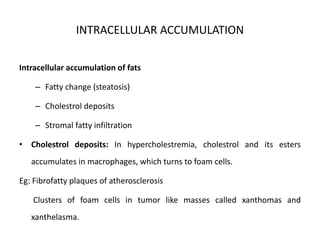
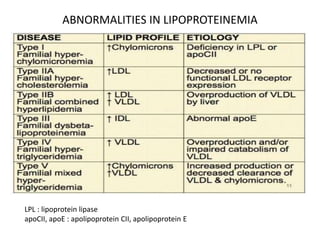
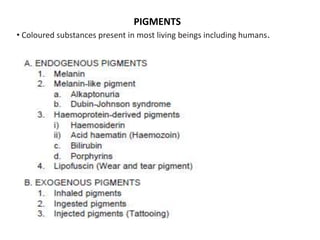
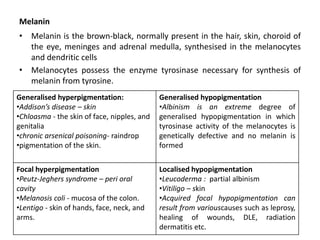
This document discusses various types of cell death and intracellular accumulations. It describes apoptosis as programmed cell death regulated by cell division. Biochemical changes in apoptosis include chromatin condensation and phosphatidylserine exposure. Apoptosis occurs physiologically in development and involution and pathologically in disease states. Gangrene is necrosis with bacterial infection and can be dry, wet, or gas gangrene. Intracellular accumulations include lipids, proteins, glycogen, and pigments. Glycogen storage diseases result from glycogen breakdown defects. Lipoprotein abnormalities can cause hyperlipidemias. Endogenous pigments include melanin while exogenous pigments come from external sources like pollution, diet, or tattoos.