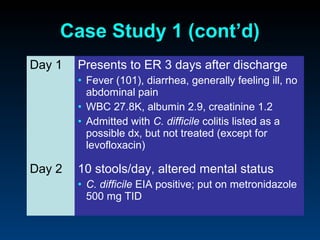
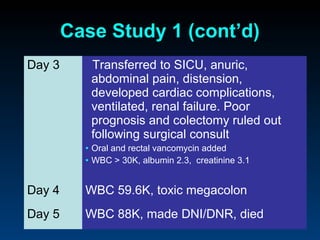
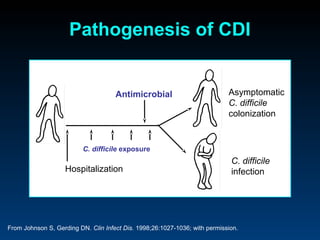
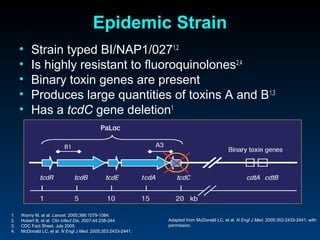
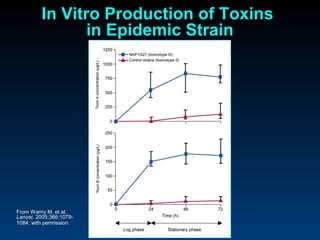
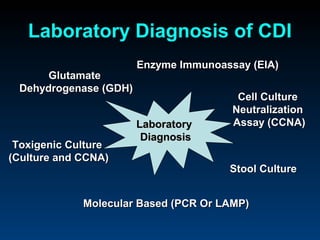
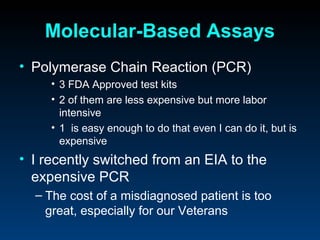
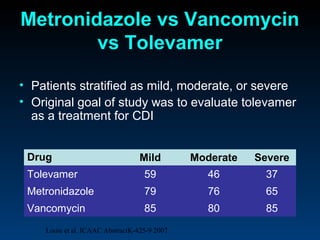
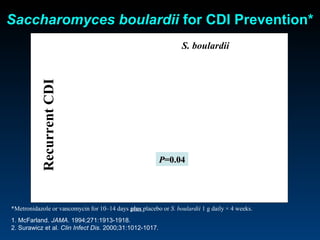
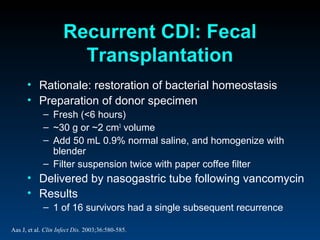
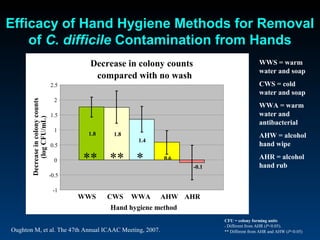
C. difficile is a spore-forming, toxin-producing bacterium that can cause severe diarrhea and life-threatening complications. While historically most cases were mild, beginning in 2000 morbidity and mortality increased, potentially due to the emergence of a hypervirulent strain. Laboratory diagnosis has been challenging due to limitations of enzyme immunoassays and the need for multi-step testing algorithms. Newer molecular tests like PCR provide improved accuracy and allow for timely treatment decisions.