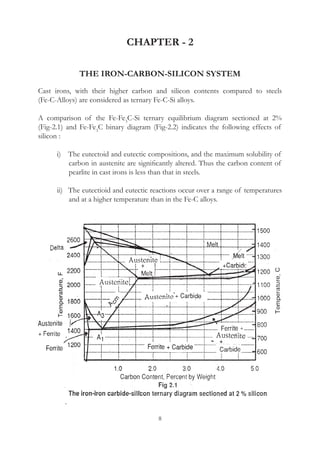
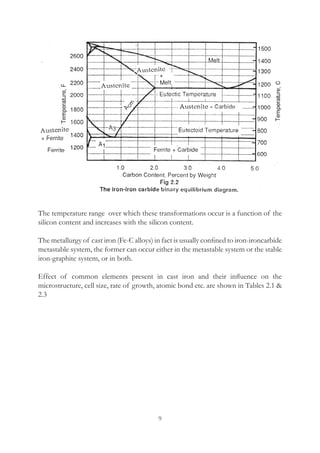
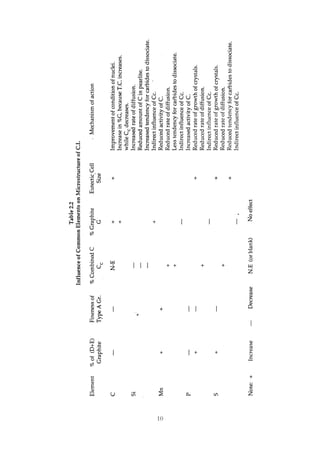
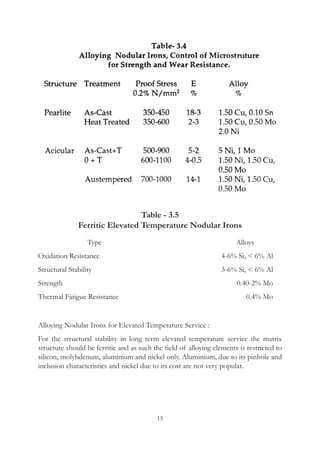
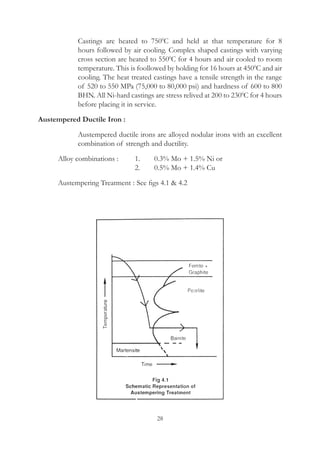
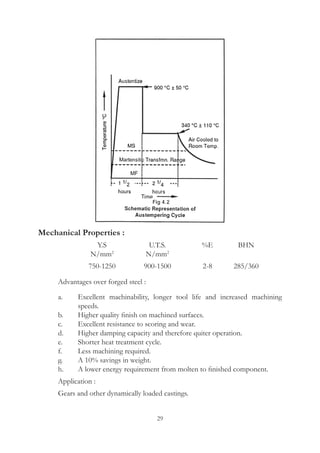
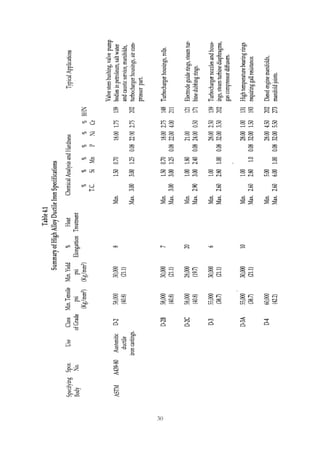
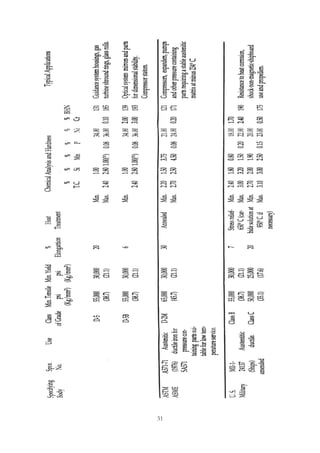
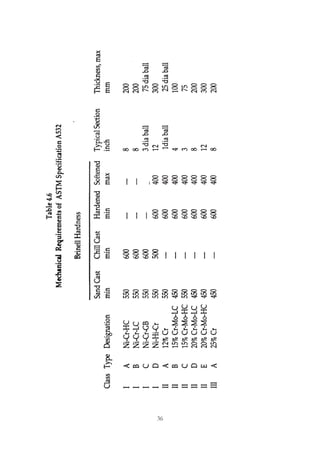
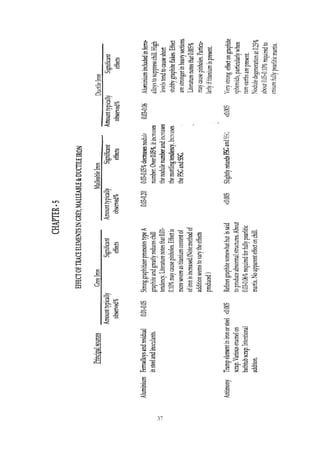
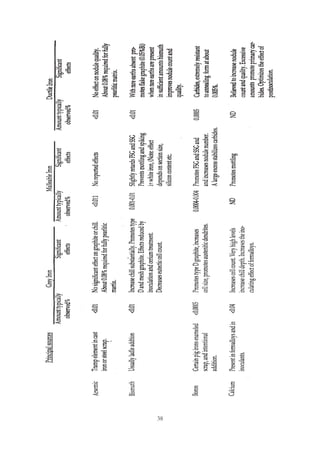
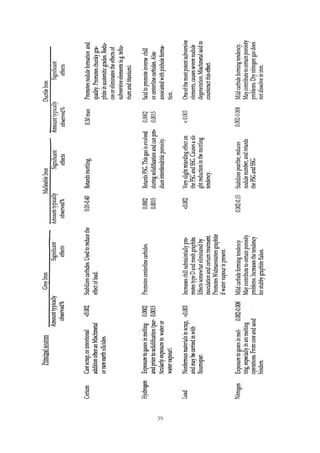
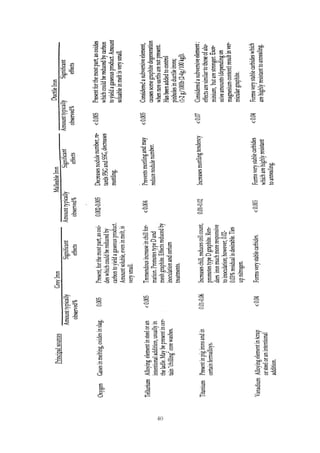
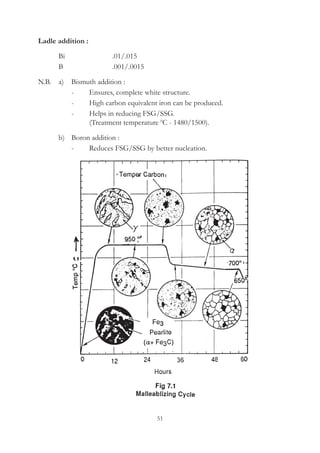
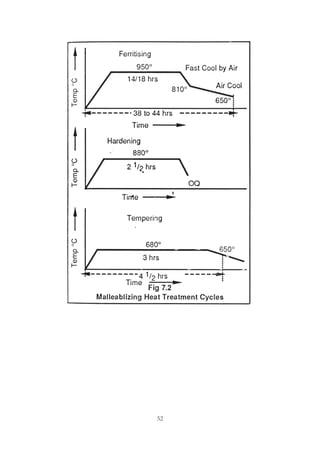
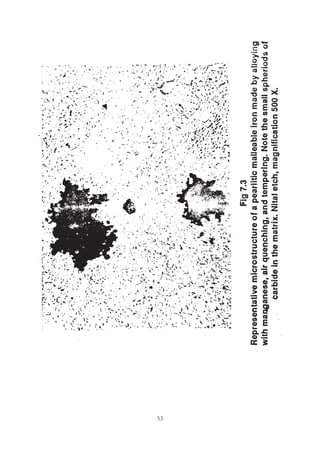
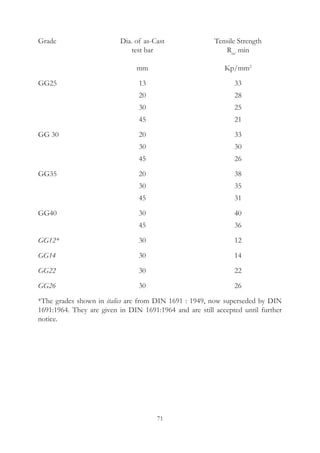
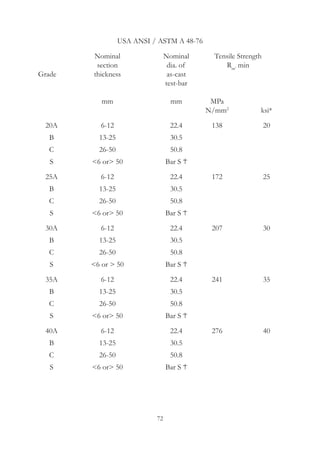
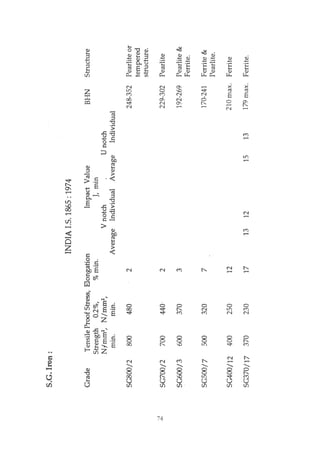
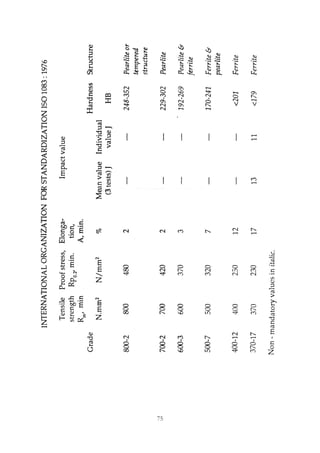
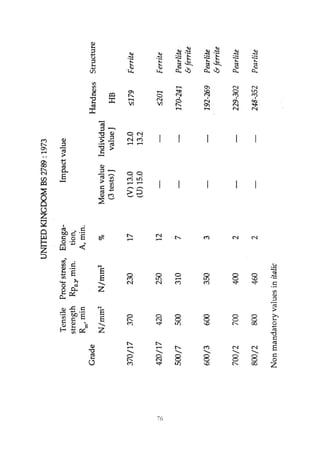
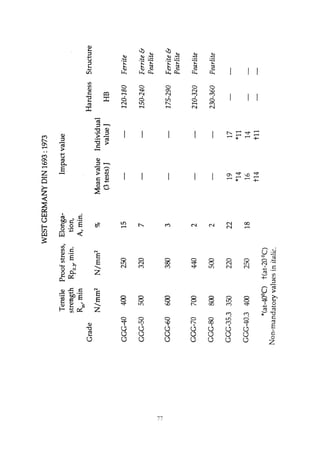
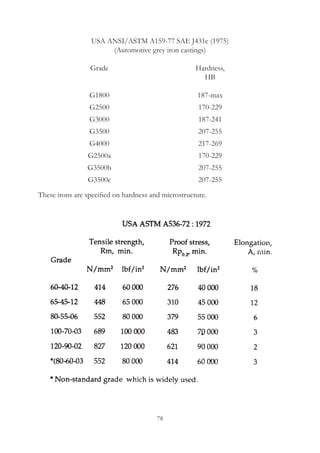
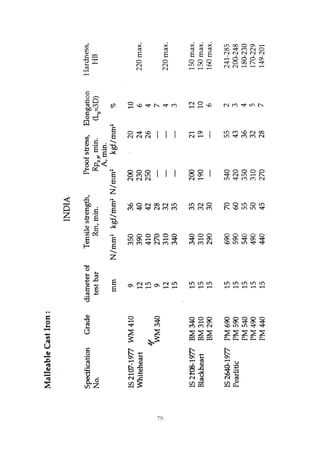
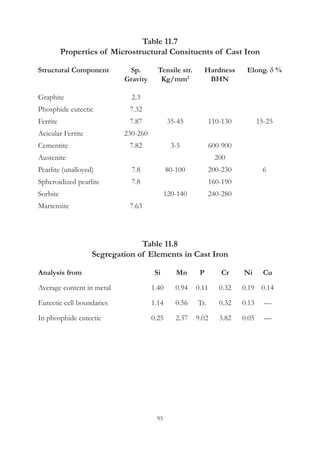
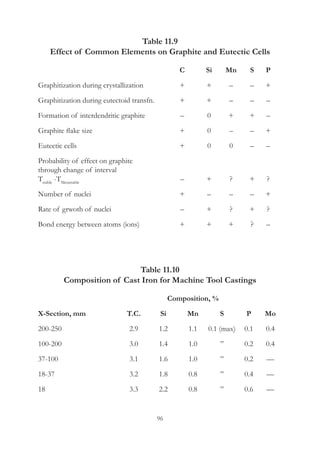
This document provides an overview of alloying elements in cast iron and their effects. Key points:
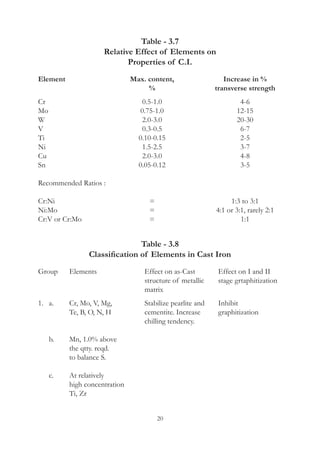
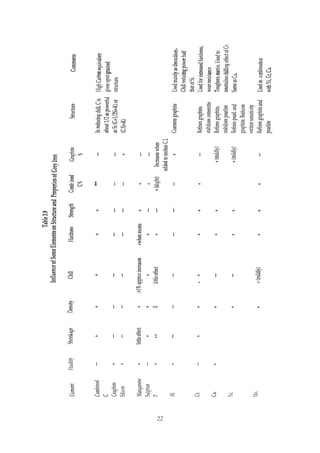
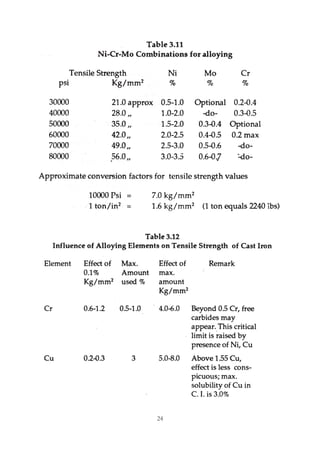
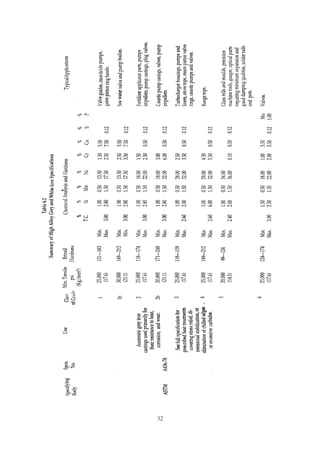
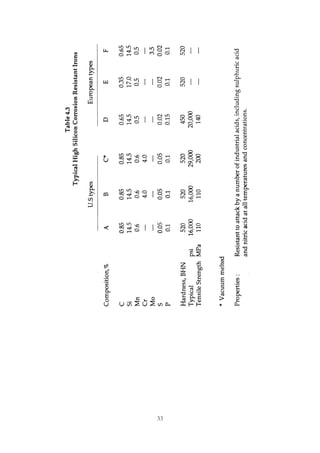
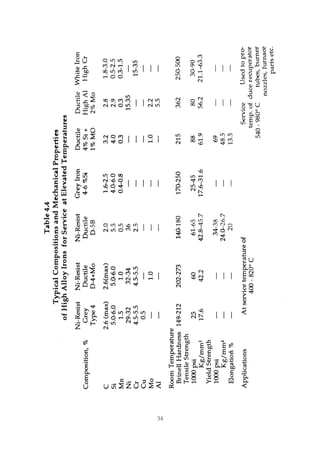
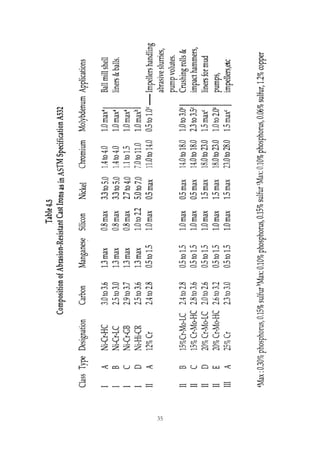
1. Common alloying elements added to cast iron include copper, nickel, chromium, molybdenum, and vanadium. These improve properties like tensile strength, hardness, wear resistance, and creep resistance.
2. Optimal levels are around 1% copper, 1% nickel, 0.4% chromium, 0.5% molybdenum, and 0.2% vanadium. Alloy combinations can have synergistic effects.
3. Nodular irons are commonly alloyed with copper, nickel, molybdenum, and tin. Ferritic nodular


















































![99
Their mutual relationship are as follows :
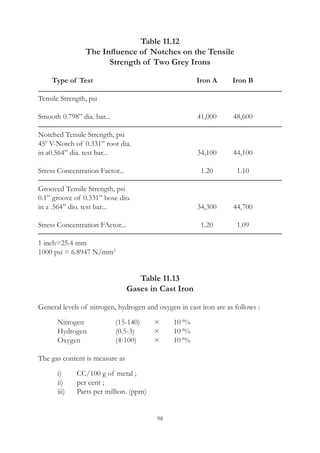
1 CC/100 g. of N2
= 0.00125%
= 12.5 ppm
1 CC/100 g. of H2
= 0.00009%
= 0.9 ppm
1 CC/100 g. of O2
= 0.00143%
= 14.3 ppm
Solubility of gases in molten cast iron :
Nitrogen
Log [n%] = -100/T=0.86-0.06 [Si+S] -0.24 C
-0.15 P + 0.015 Mn + 0.03 Cr
Hydrogen
For the normal compositions and temperatures and at atmospheric pressure :
[H] CC/100g = 25 - 3.5 C - 2Si + 10 Mn - 3 Cr.
However, in hypereutectic cast irons, carbon increases the solubility of H2
due to
adsorption on graphite.
Osygen
Log [%0] = -2975/T - 1.06 -log[%C] + 0.19 [%C]
- 0.5 log [% Si]
Like in the case of hydrogen, the solubility of oxygen in hypereutectic irons also
increases due to adsorption.](https://image.slidesharecdn.com/castiron-210805043635/85/Cast-iron-99-320.jpg)