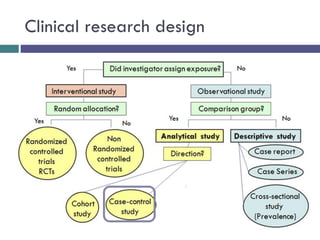
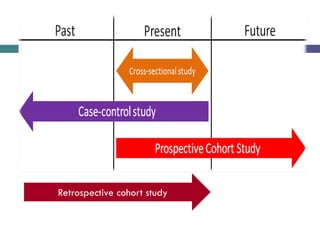
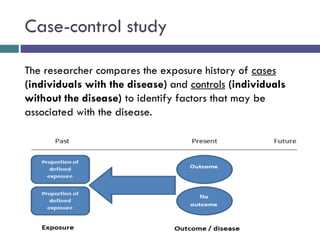
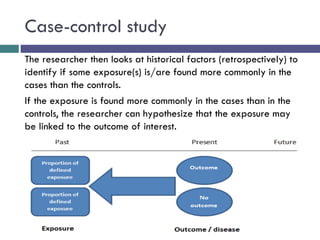
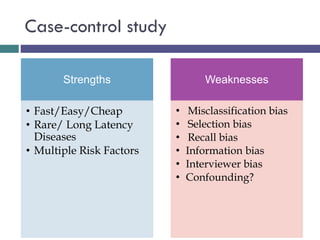
This document discusses case-control studies, a type of observational research focused on identifying associations between exposures and diseases through retrospective comparisons of cases and controls. It outlines potential biases in the selection of cases and controls, highlights the importance of precise definitions to avoid misclassification, and describes the interpretation of odds ratios as a measure of association. The document also emphasizes the strengths and weaknesses of case-control studies and includes examples of how different studies are designed and evaluated.

![[A] Case-control studies
*A type of observational study commonly used to look
at factors associated with diseases or outcomes.
*Compare groups retrospectively.
*They are often used to generate hypotheses that can
then be studied via prospective cohort or other studies.](https://image.slidesharecdn.com/case-controlstudies-240430104257-918bc2ca/85/Case-control-studies-drug-information-lec-4-320.jpg)
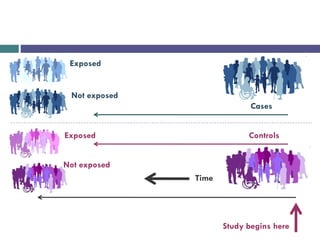
![Example:
Study begins here
Controls [No peptic ulcer]
Cases [Peptic ulcer]
Time
NSAIDs use
NSAIDs use
Not NSAIDs use
No NSAIDs use](https://image.slidesharecdn.com/case-controlstudies-240430104257-918bc2ca/85/Case-control-studies-drug-information-lec-9-320.jpg)
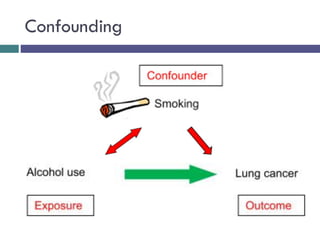
![[B] Bias & validity of case control study](https://image.slidesharecdn.com/case-controlstudies-240430104257-918bc2ca/85/Case-control-studies-drug-information-lec-10-320.jpg)




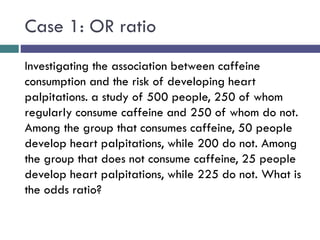
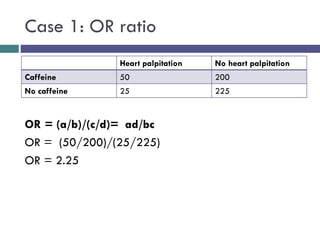
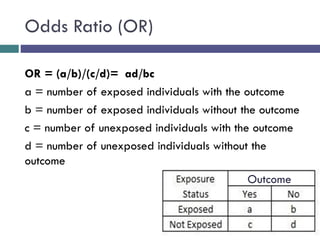
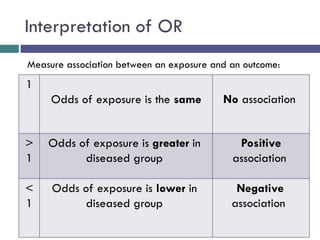
![[C] Odds Ratio (OR)
*The odds ratio is commonly used in case-control studies
*Where the odds of exposure are compared between
cases (individuals with the outcome) and controls
(individuals without the outcome)
*To estimate the strength of association between the
exposure and the outcome.](https://image.slidesharecdn.com/case-controlstudies-240430104257-918bc2ca/85/Case-control-studies-drug-information-lec-20-320.jpg)
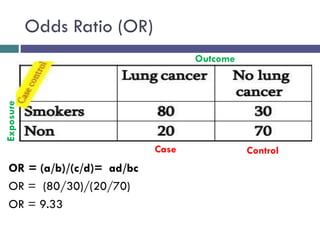
![[D] Cases](https://image.slidesharecdn.com/case-controlstudies-240430104257-918bc2ca/85/Case-control-studies-drug-information-lec-24-320.jpg)