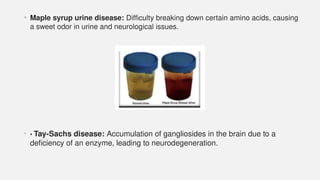
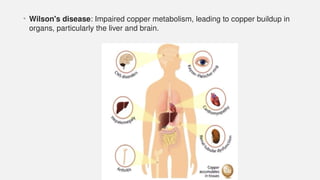
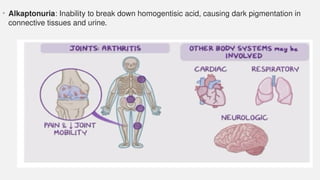
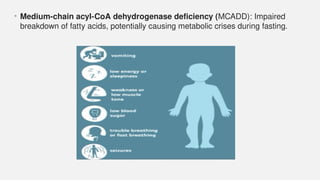
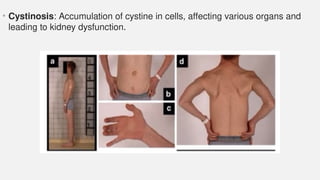
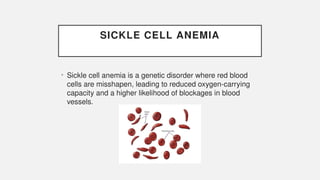
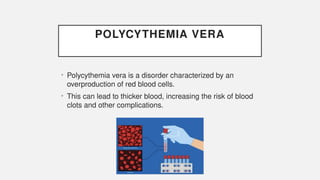
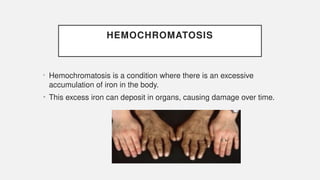
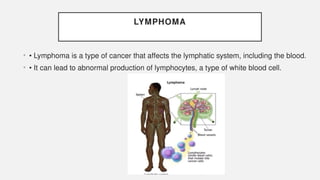
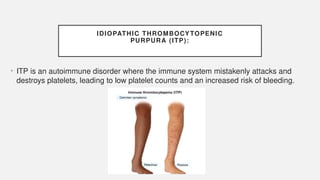
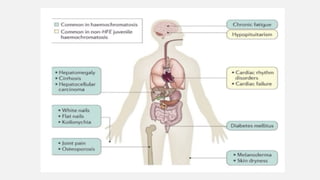
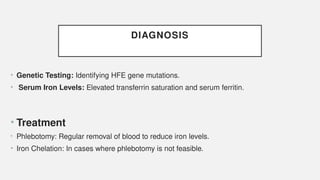
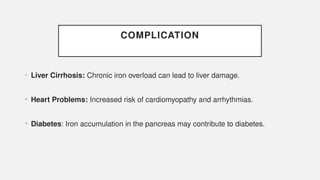
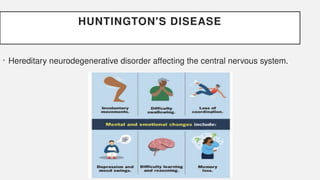
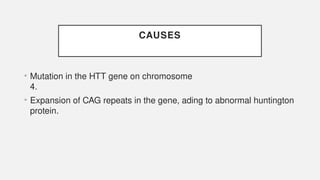
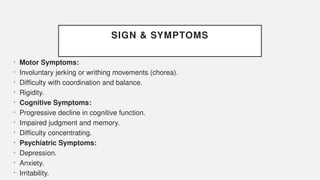
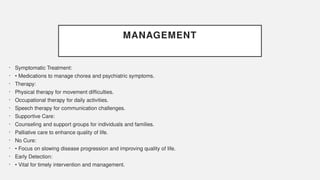
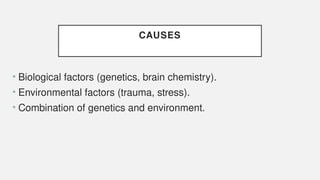
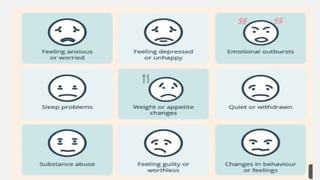
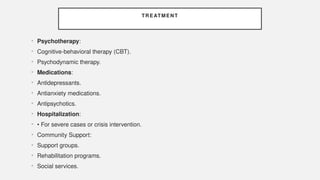
The document discusses various genetic disorders, including familial cancer syndromes, inborn errors of metabolism, blood group alleles, hematological disorders, and mental illnesses. It covers definitions, key concepts, implications for family members, risk-reducing strategies, and treatment approaches for conditions such as Huntington's disease, anemia, and hemochromatosis. Emphasis is placed on genetic testing, prevention, collaboration among specialists, and the management of symptoms.