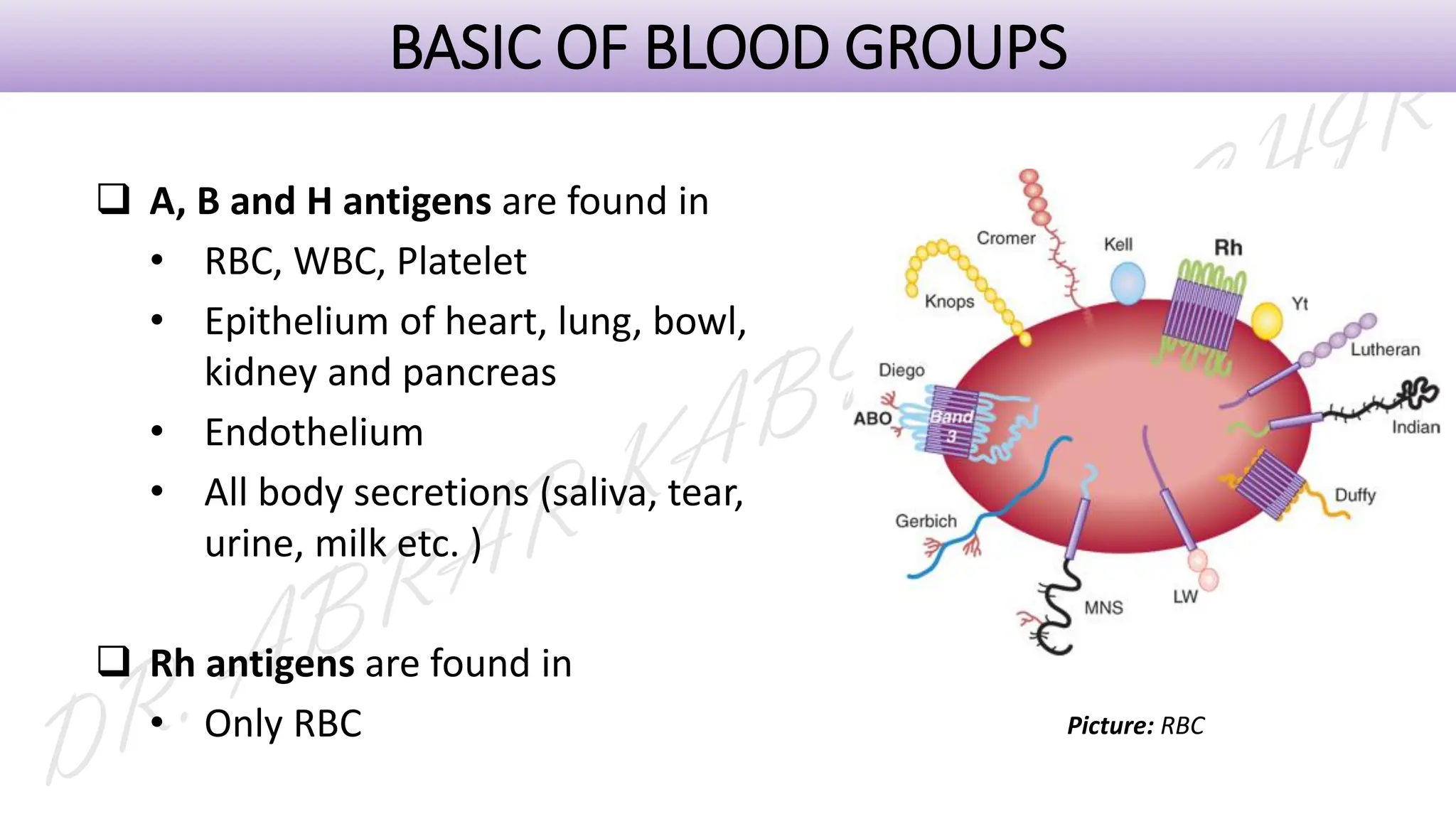
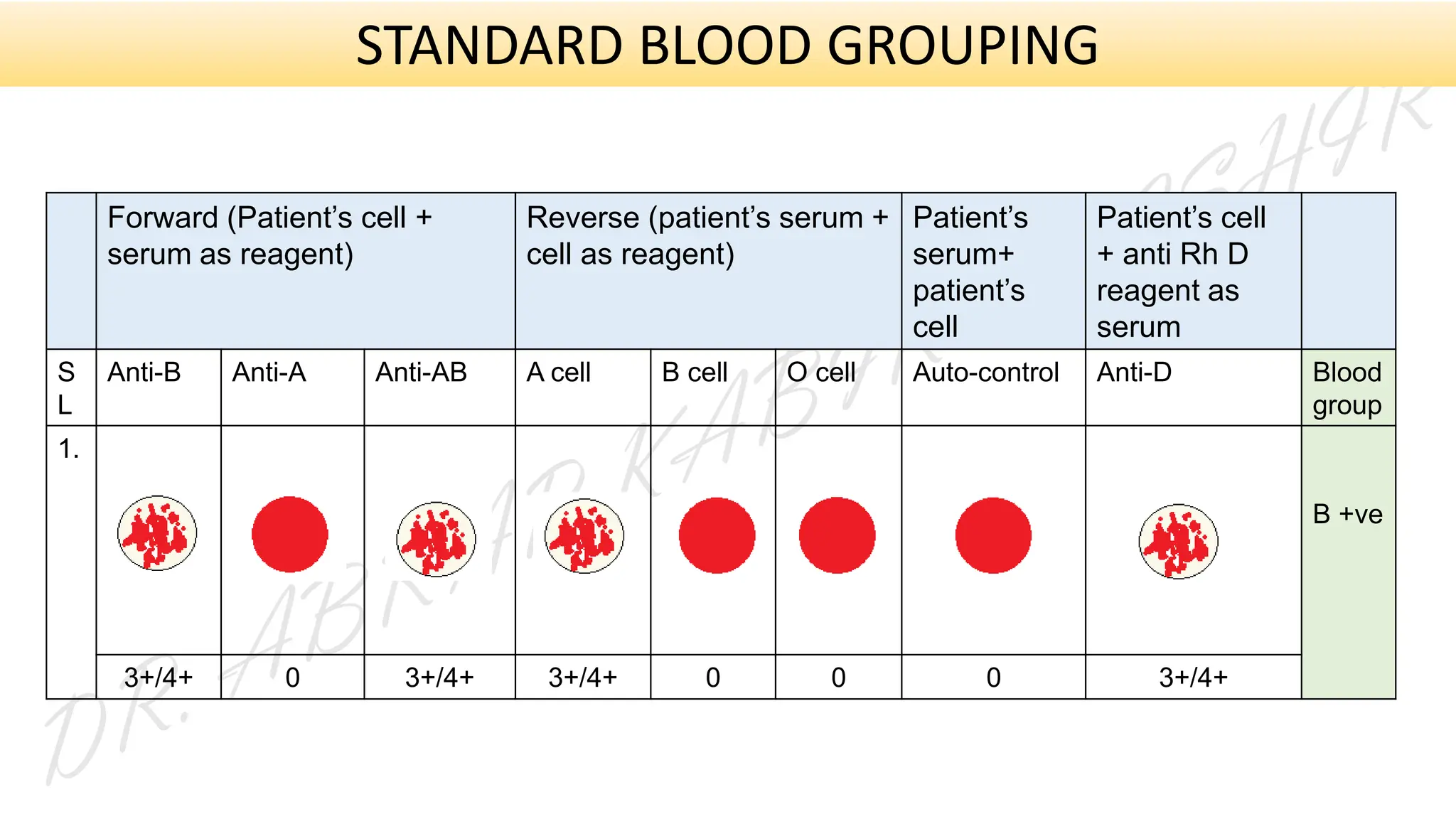
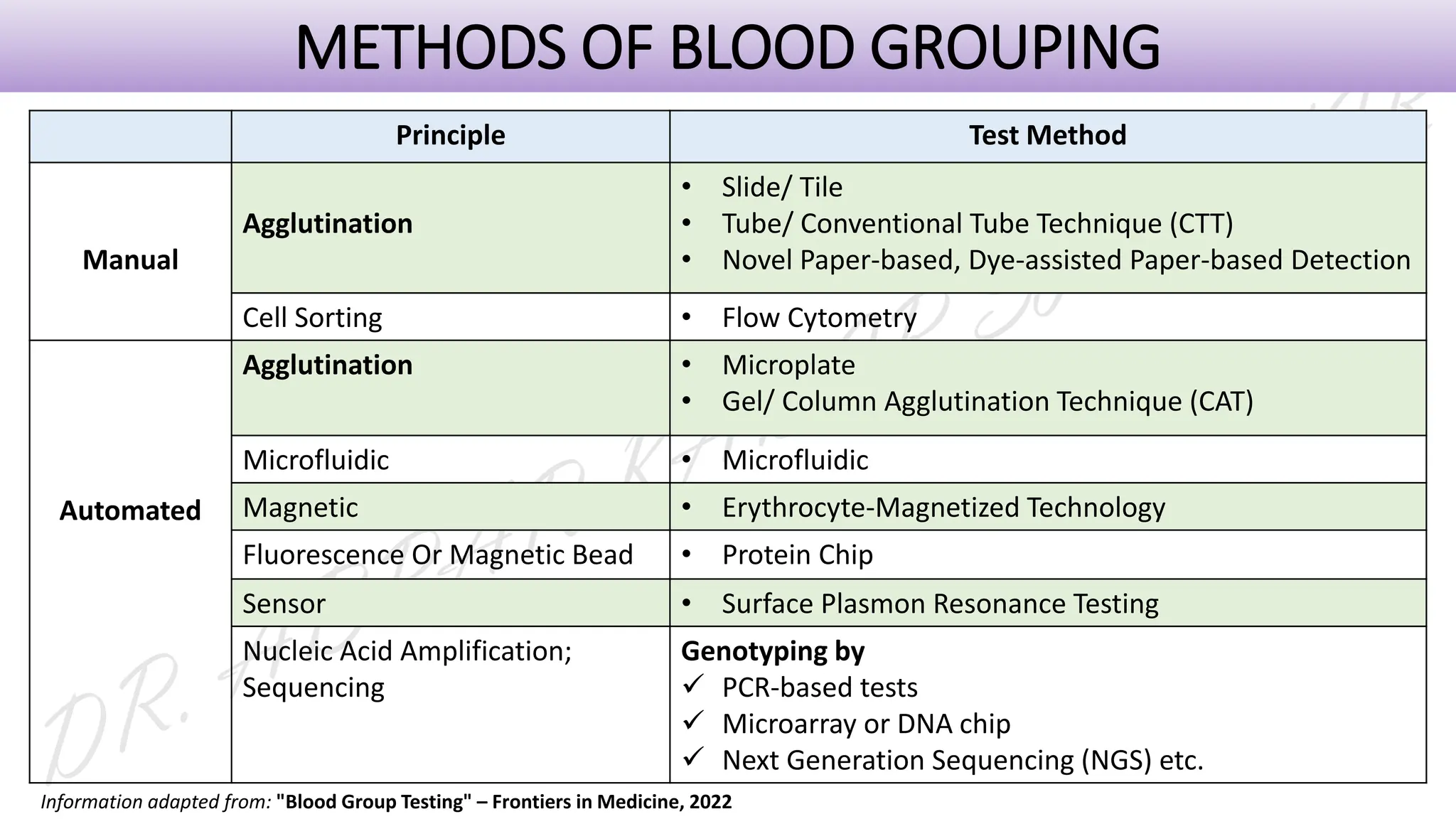
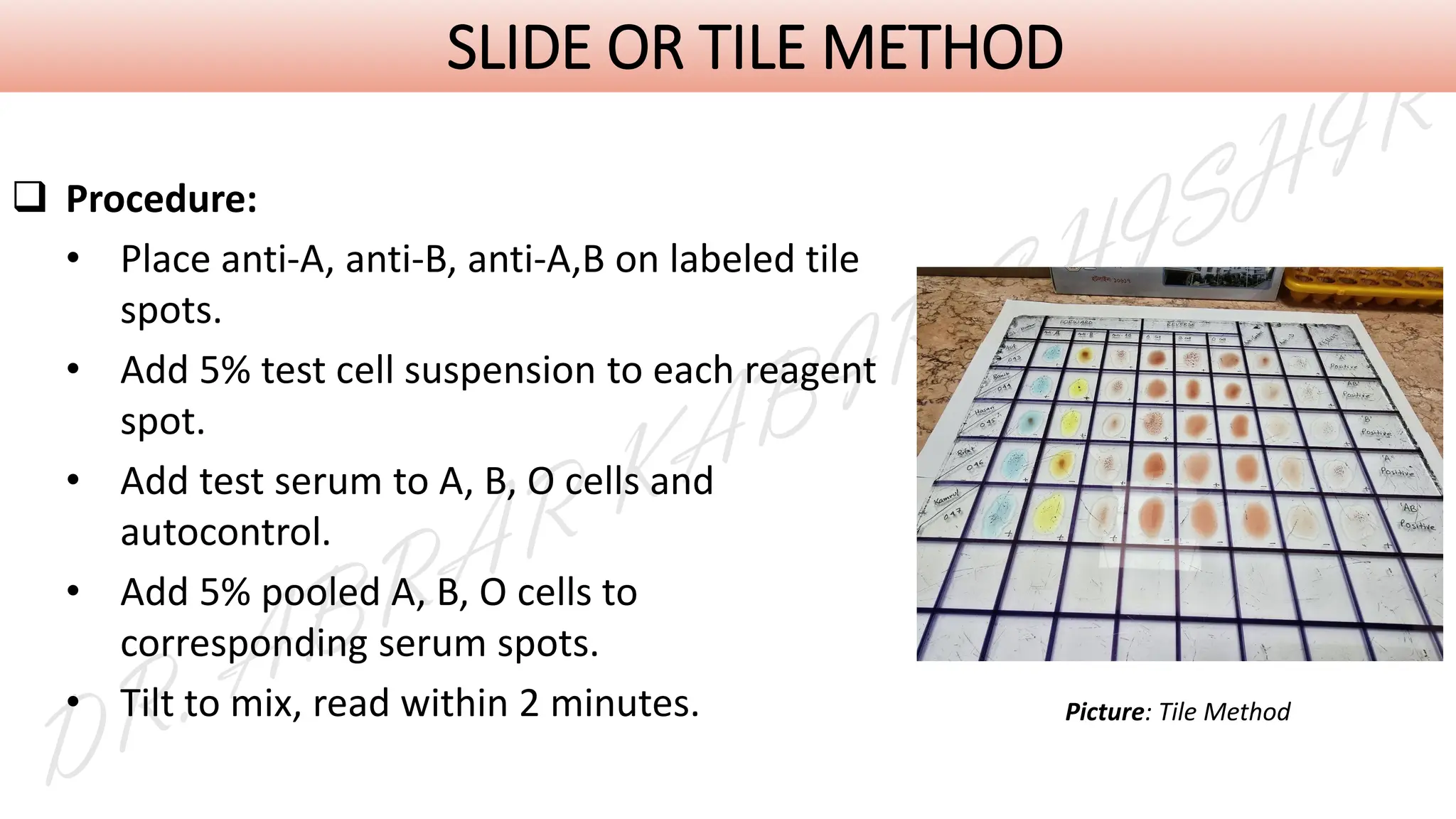
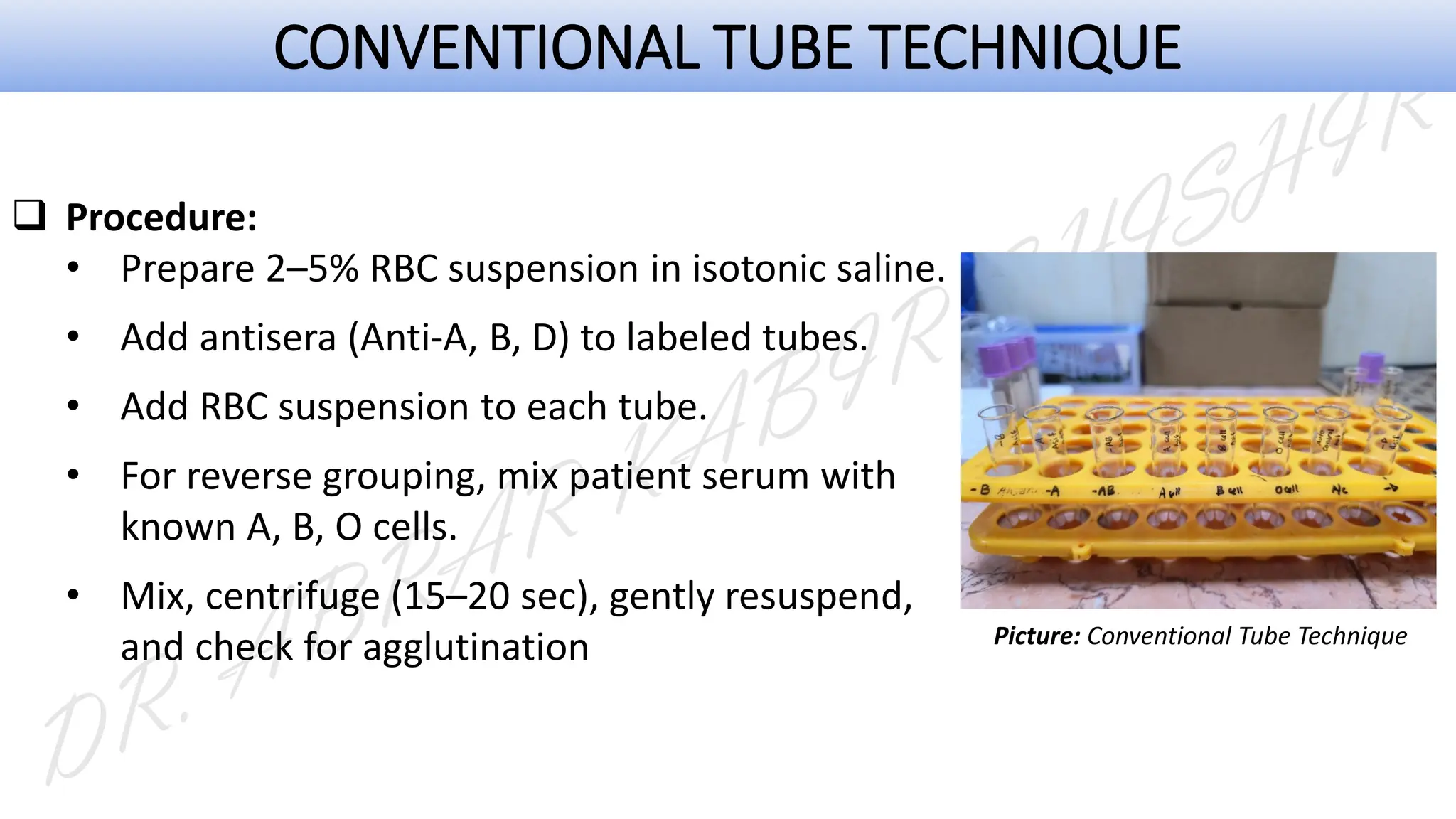
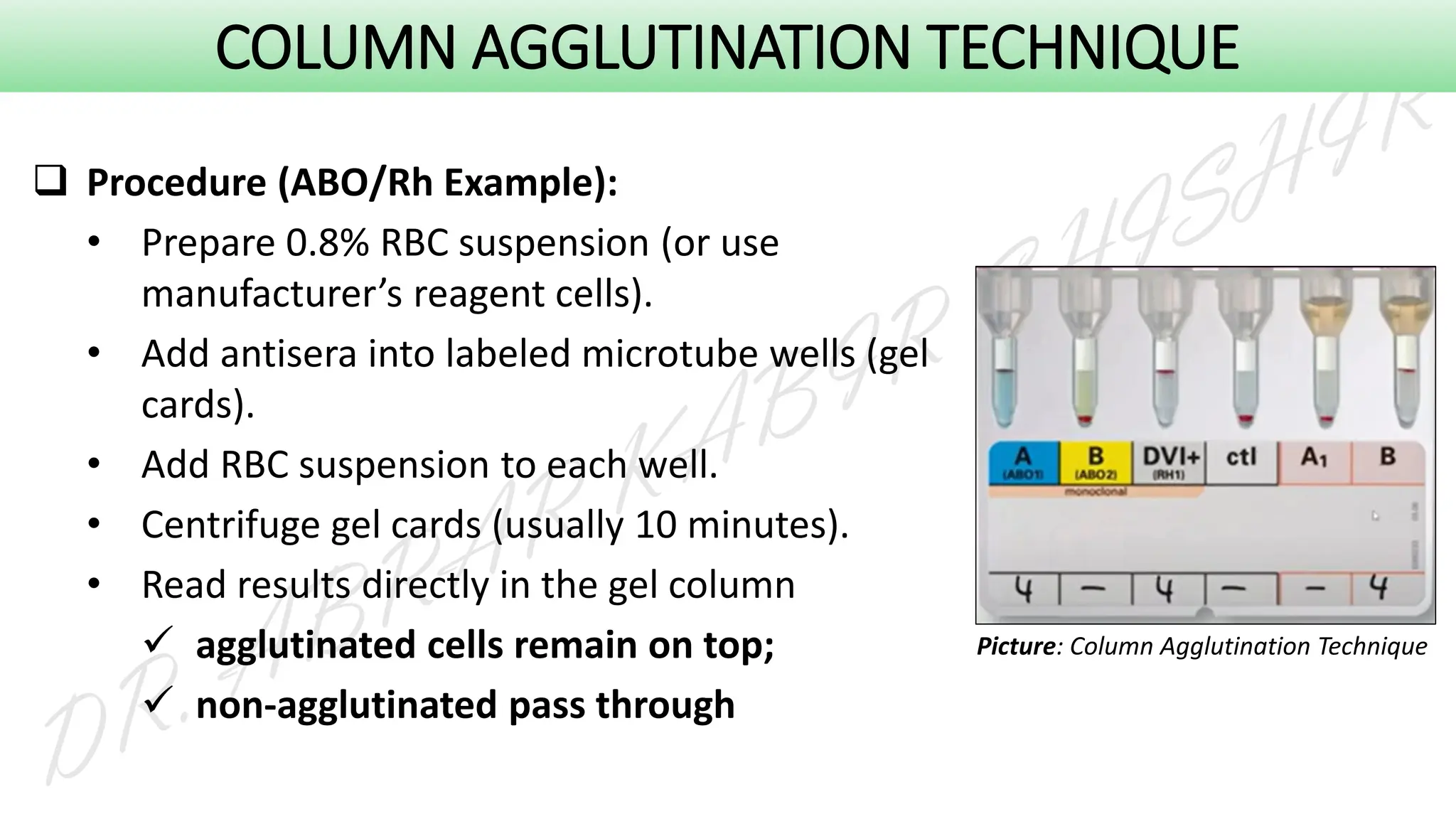
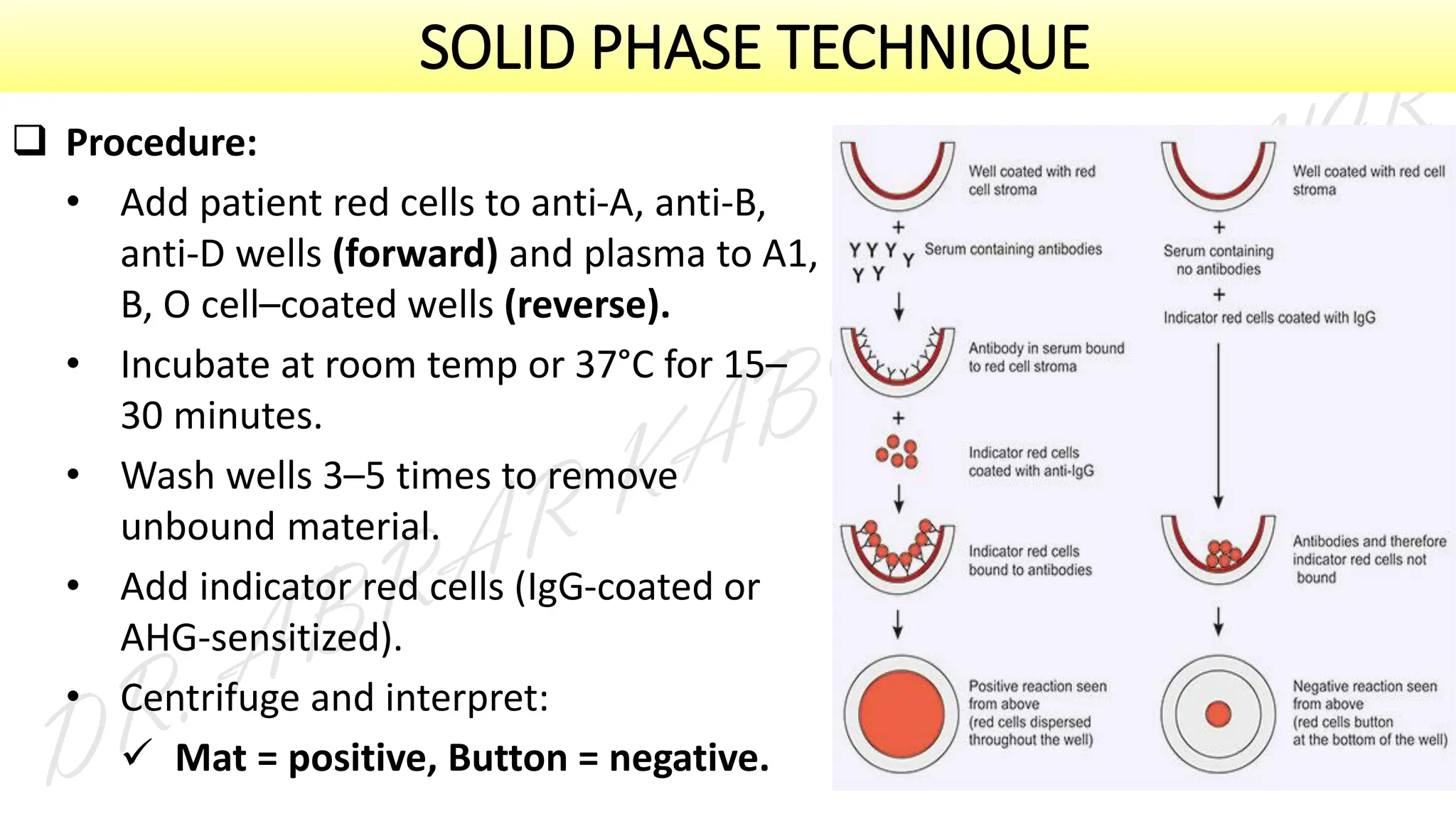
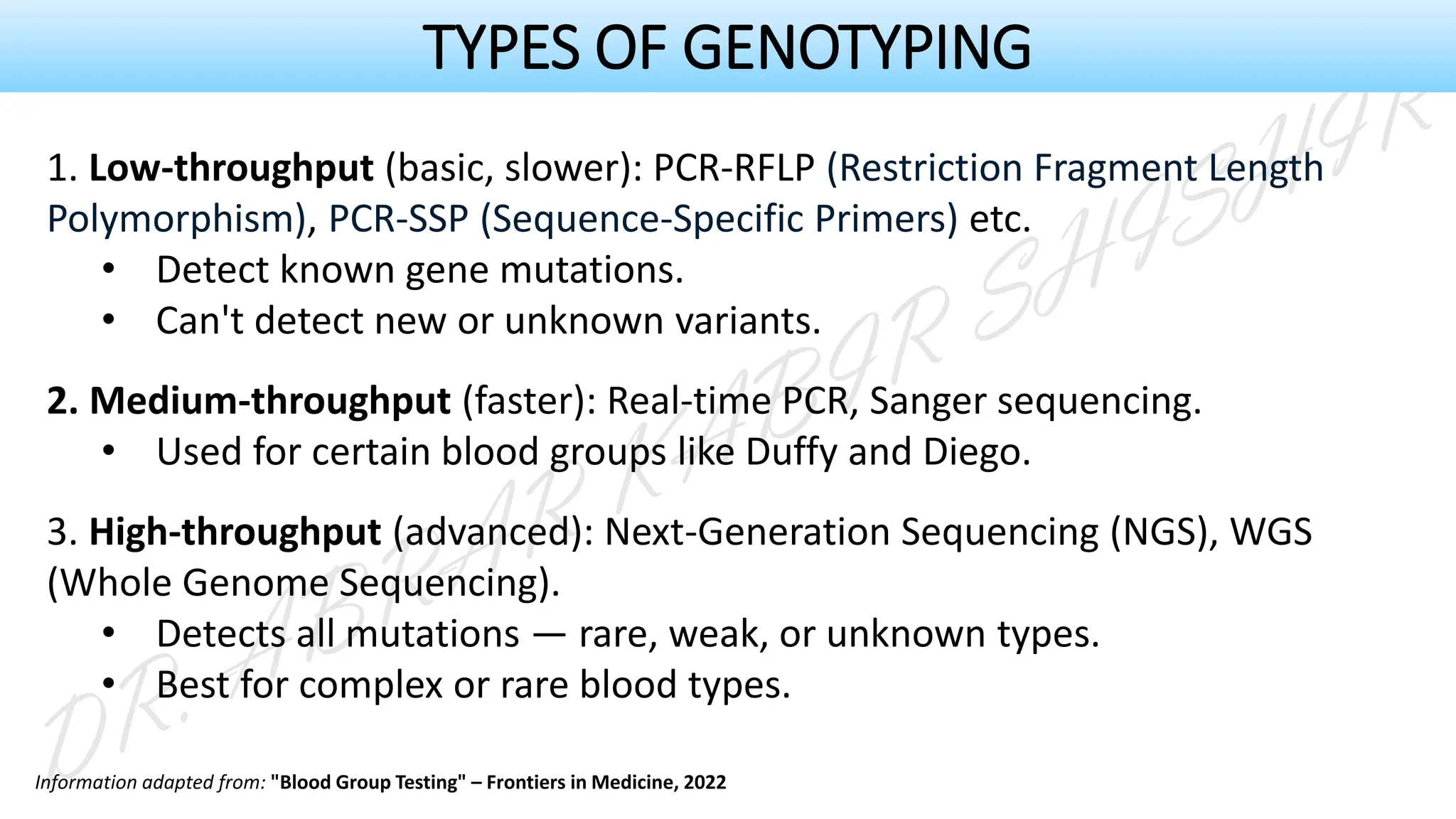
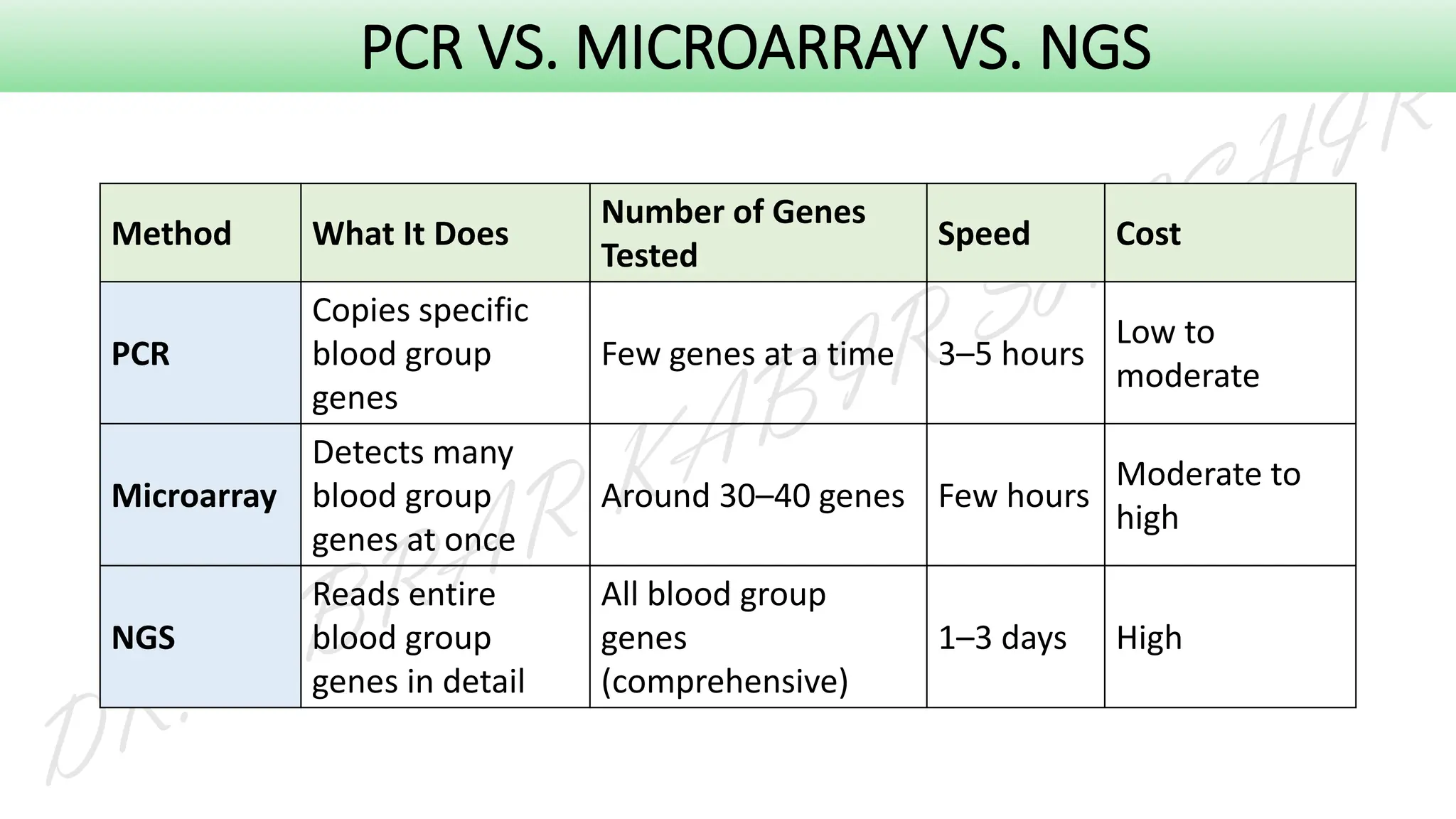
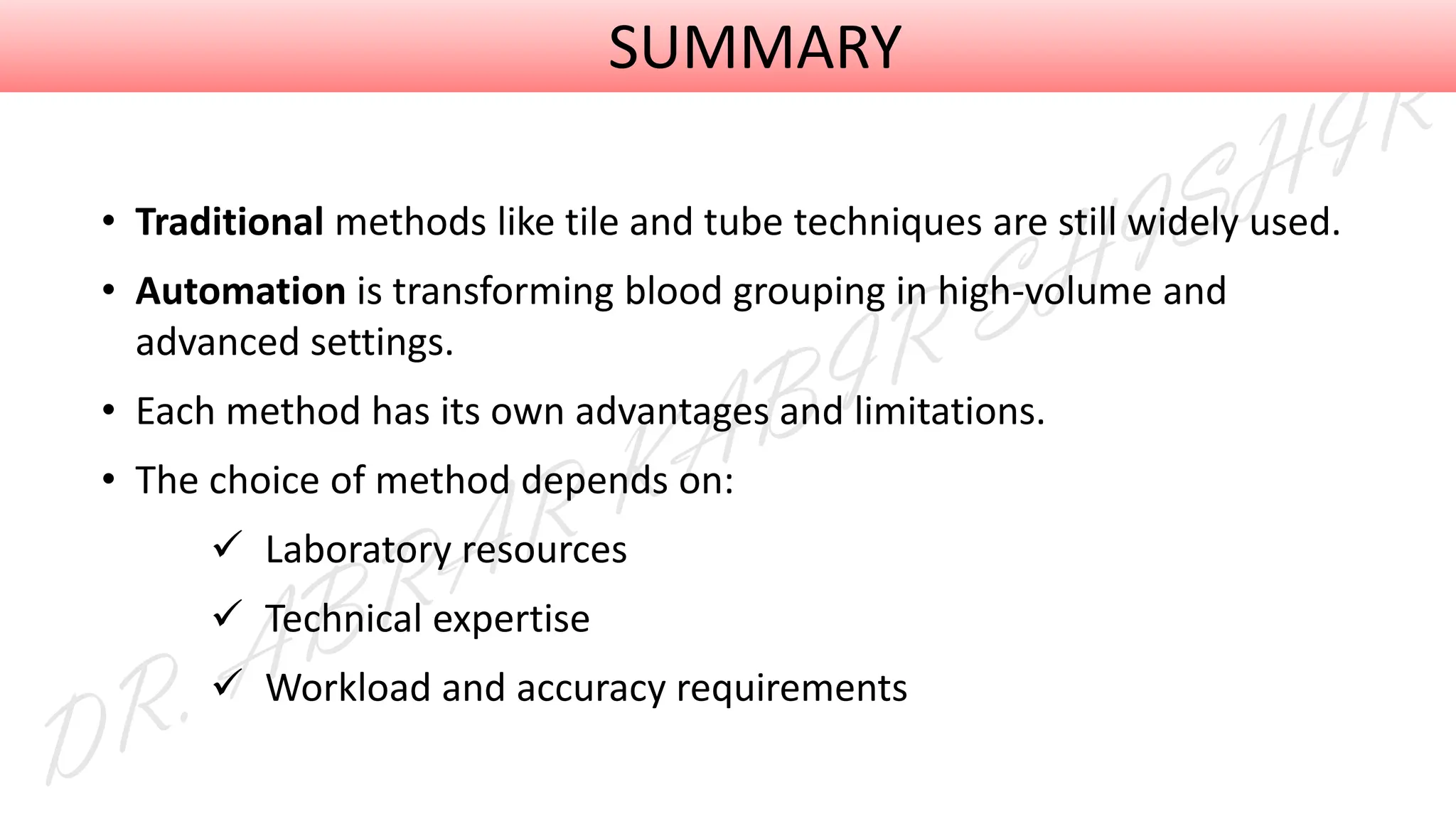
This presentation explores various blood grouping methods, including traditional serological techniques like slide, tube, and microplate methods, as well as advanced methods such as gel card technology, solid-phase systems, and molecular genotyping. It highlights their principles, applications, and challenges in clinical and blood bank settings. Ideal for medical students, laboratory professionals, and transfusion medicine specialists, this presentation offers a concise yet comprehensive overview of ABO and Rh typing practices in modern transfusion services.



![ISBT system
symbol/ Name
(Number)
Gene Name:
ISBT (HGNC)
Chromosome
Location
Associated Blood Group
Antigens [Null phenotype]
ABO (001) ABO(ABO) 9q34.2 A; B; A,B; A1 [Group O]
RH/ Rh (004) RH (RHD,
RHCE)
1q36.11 D, G, C, E, c, e, V, VS and more
[Rh null]
H(018) H (FUT1) 19q13.33 H [Bombay (Oh)]
BASIC GENETICS OF BLOOD GROUPS](https://image.slidesharecdn.com/bloodgroupingmethodsbydr-250731051212-b4612fa7/75/BLOOD-GROUPING-METHODS-by-Dr-Abrar-Kabir-Shishir-pdf-4-2048.jpg)