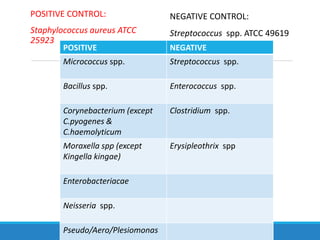
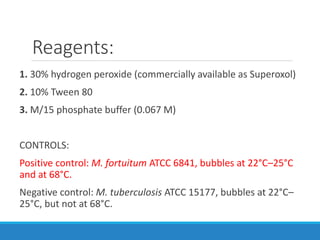
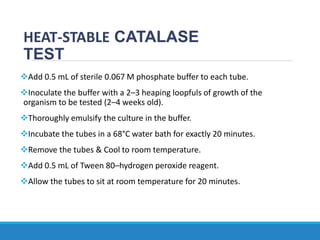
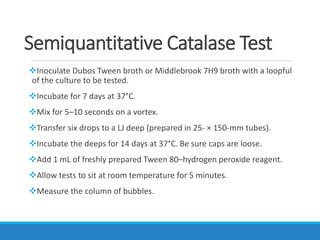
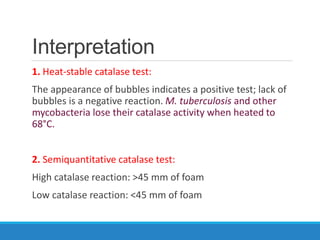
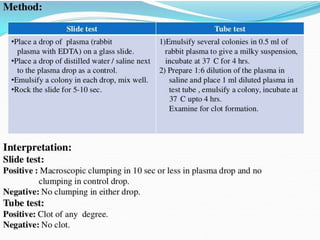
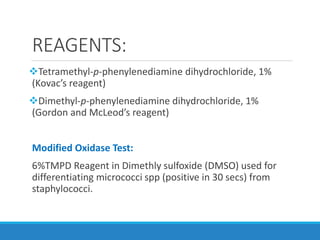
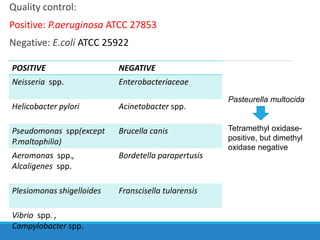
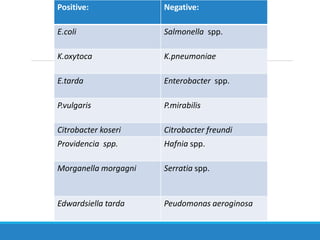
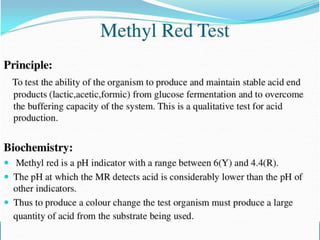
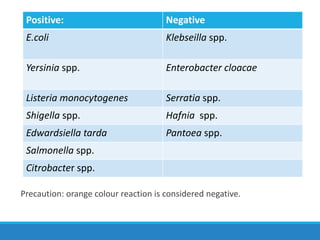
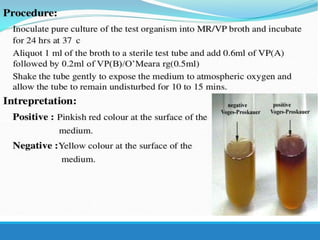
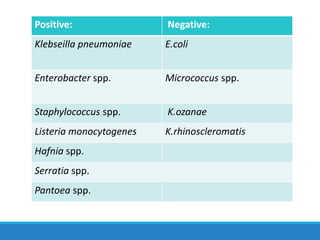
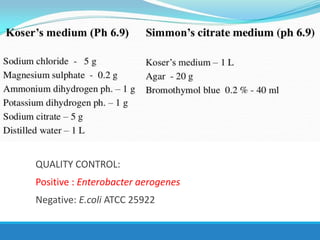
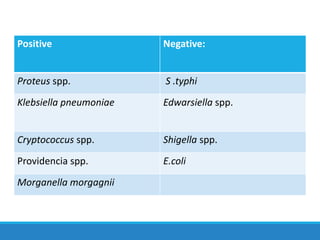
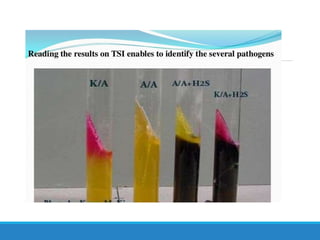
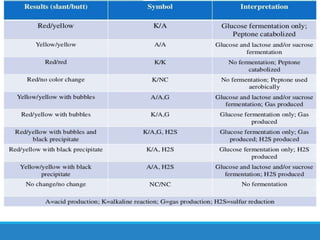
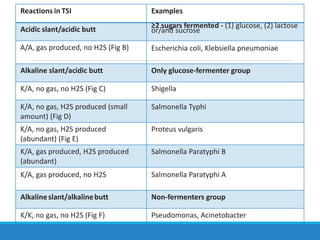
This document provides information on various biochemical tests used to identify microorganisms. It describes tests such as catalase, coagulase, oxidase, triple sugar iron, urease, citrate, and indole. Optimal conditions, reagents, controls, and interpretation of results are outlined for each test. A variety of microorganisms that produce positive and negative reactions are listed. The document aims to guide the identification of organisms based on their biochemical characteristics and reactions.