This document provides an overview of atomic structure and components, including the nucleus, protons, neutrons, and electrons. It explains atomic and mass numbers, energy levels, electronic configuration, and chemical symbols, along with examples of common elements. Additionally, it includes engaging activities like quizzes and atom modeling to reinforce learning.


![Parts of an Atom
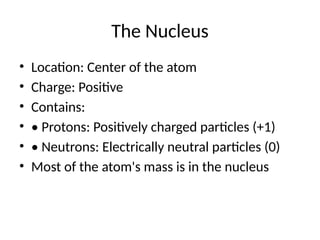
• • Nucleus: The central core of the atom
• • Electrons: Particles that orbit around the
nucleus
• [Image: Simple diagram of an atom with
labeled nucleus and electrons]](https://image.slidesharecdn.com/atomicstructurepresentation-250122233207-4f1b18cb/85/Atomic_Structure_and-elements-Presentation-pptx-3-320.jpg)
![Electrons
• Orbit around the nucleus at high speed
• Negatively charged particles (-1)
• Very small mass compared to protons and
neutrons
• Number of electrons equals number of
protons in a neutral atom
• [Image: Illustration of electrons orbiting
nucleus]](https://image.slidesharecdn.com/atomicstructurepresentation-250122233207-4f1b18cb/85/Atomic_Structure_and-elements-Presentation-pptx-5-320.jpg)
![Energy Levels
• Imaginary regions where electrons move
around the nucleus
• Maximum of seven energy levels in the largest
atoms
• Levels are labeled K, L, M, N, O, P, Q (or 1, 2, 3,
4, 5, 6, 7)
• [Image: Diagram of atom with multiple energy
levels]](https://image.slidesharecdn.com/atomicstructurepresentation-250122233207-4f1b18cb/85/Atomic_Structure_and-elements-Presentation-pptx-7-320.jpg)
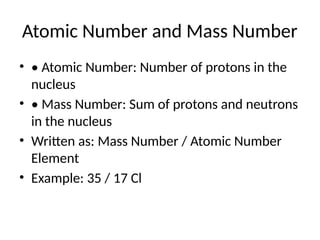
![Electronic Configuration Example
• Let's distribute electrons for 35 / 17 Cl:
• • 17 electrons total
• • K: 2 electrons
• • L: 8 electrons
• • M: 7 electrons
• [Image: Chlorine atom with electron
distribution]](https://image.slidesharecdn.com/atomicstructurepresentation-250122233207-4f1b18cb/85/Atomic_Structure_and-elements-Presentation-pptx-9-320.jpg)
![Common Elements and Their
Symbols
• Hydrogen: H, Oxygen: O, Carbon: C, Nitrogen:
N, Chlorine: Cl, Helium: He
• [Image: Periodic table highlighting these
elements]](https://image.slidesharecdn.com/atomicstructurepresentation-250122233207-4f1b18cb/85/Atomic_Structure_and-elements-Presentation-pptx-11-320.jpg)

![Fun Fact: Element Hunt
• Can you find these elements around you?
• - Oxygen (in the air)
• - Carbon (in pencils)
• - Sodium (in salt)
• - Calcium (in chalk)
• [Image: Collage of everyday items containing
these elements]](https://image.slidesharecdn.com/atomicstructurepresentation-250122233207-4f1b18cb/85/Atomic_Structure_and-elements-Presentation-pptx-13-320.jpg)
![Quick Quiz
• • What is the building block of matter?
• • Name the three main parts of an atom.
• • What does the atomic number represent?
• [Image: Question mark or thinking emoji]](https://image.slidesharecdn.com/atomicstructurepresentation-250122233207-4f1b18cb/85/Atomic_Structure_and-elements-Presentation-pptx-14-320.jpg)
![Review Game: Atomic Assembly
• In groups, create a model of an atom using
household items:
• • Nucleus: A ball or fruit
• • Protons: Red buttons or beads
• • Neutrons: White buttons or beads
• • Electrons: Small blue stickers or pins
• [Image: Example of a creative atom model
made from everyday objects]](https://image.slidesharecdn.com/atomicstructurepresentation-250122233207-4f1b18cb/85/Atomic_Structure_and-elements-Presentation-pptx-15-320.jpg)