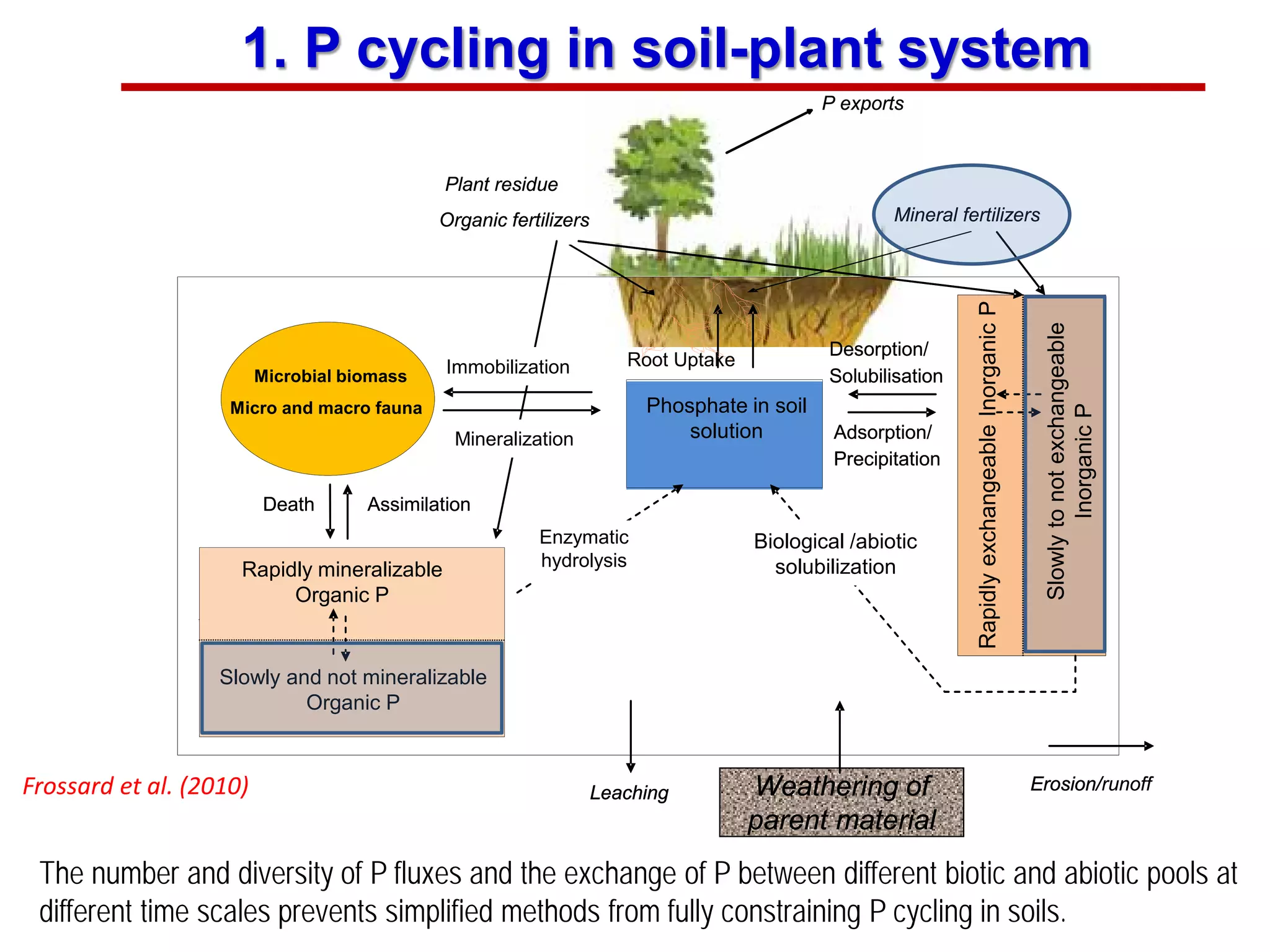
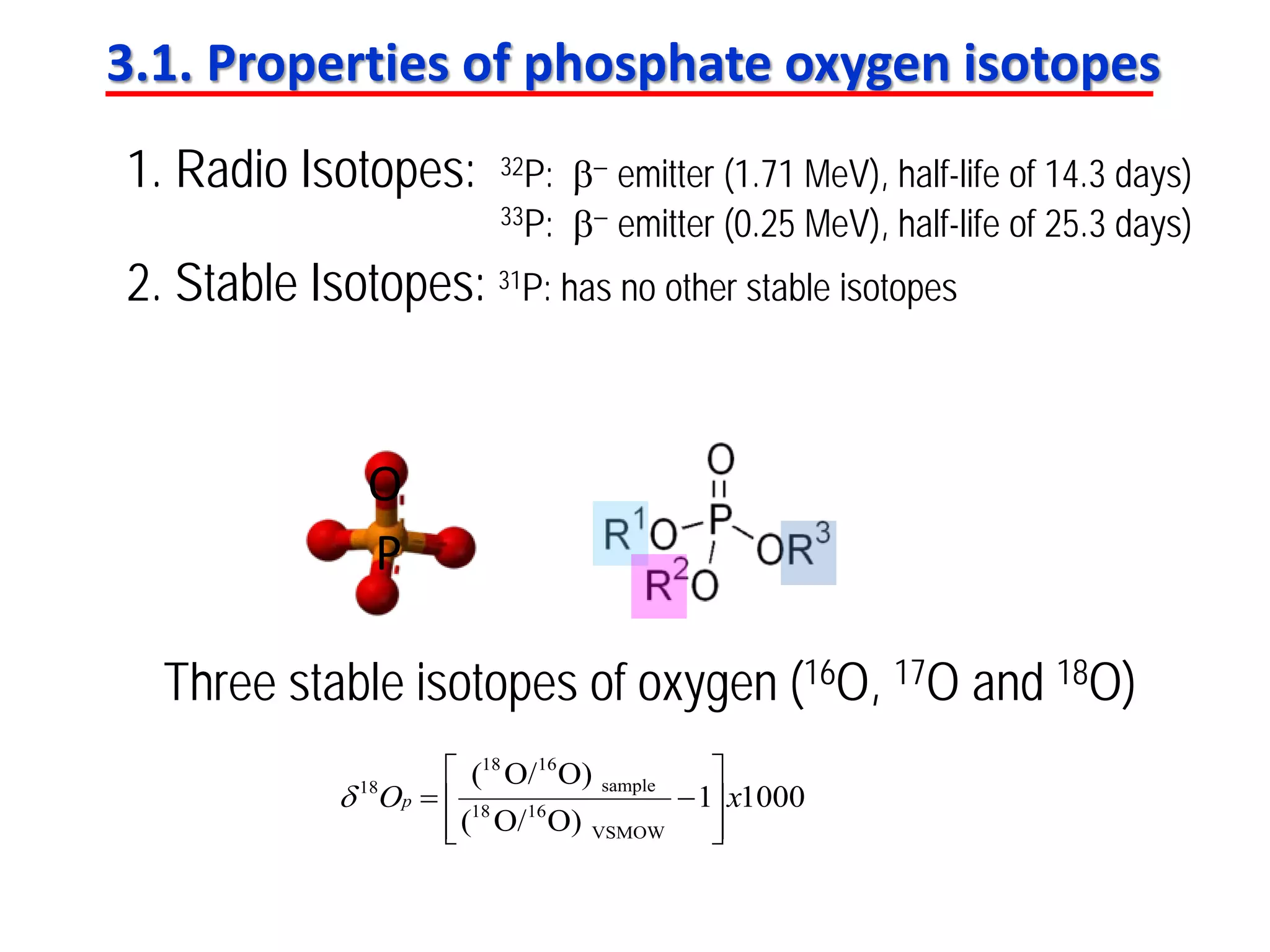
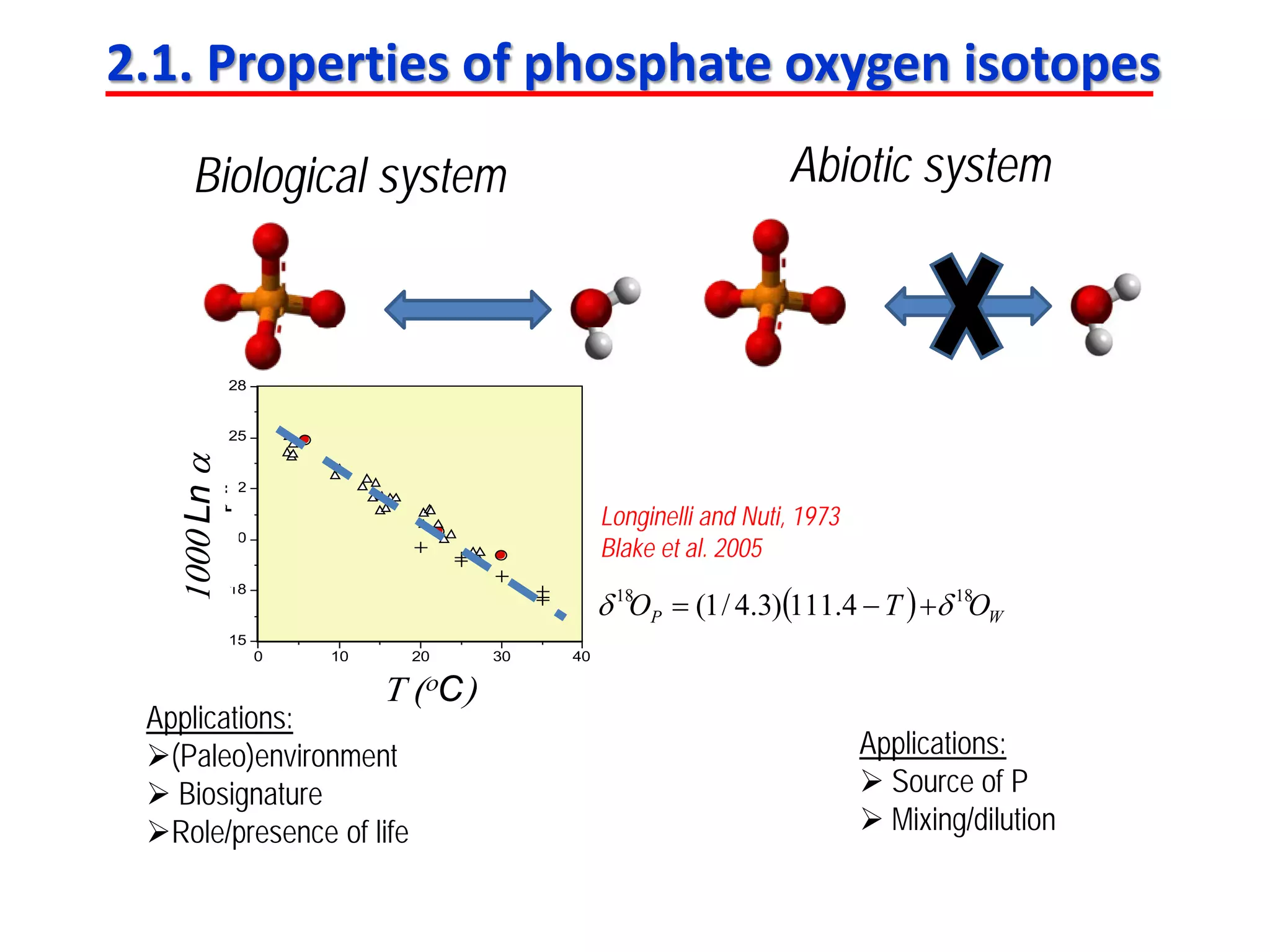
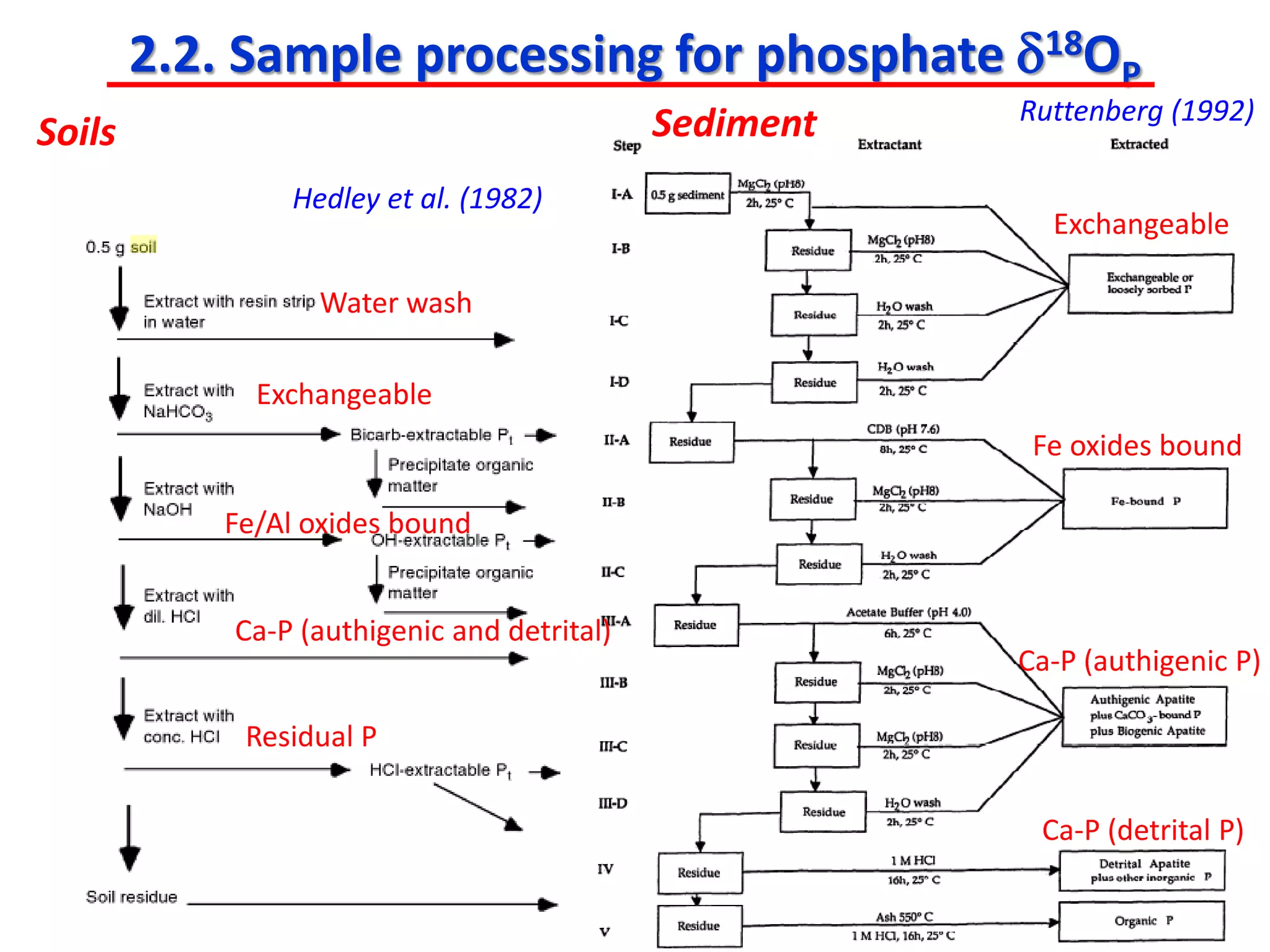
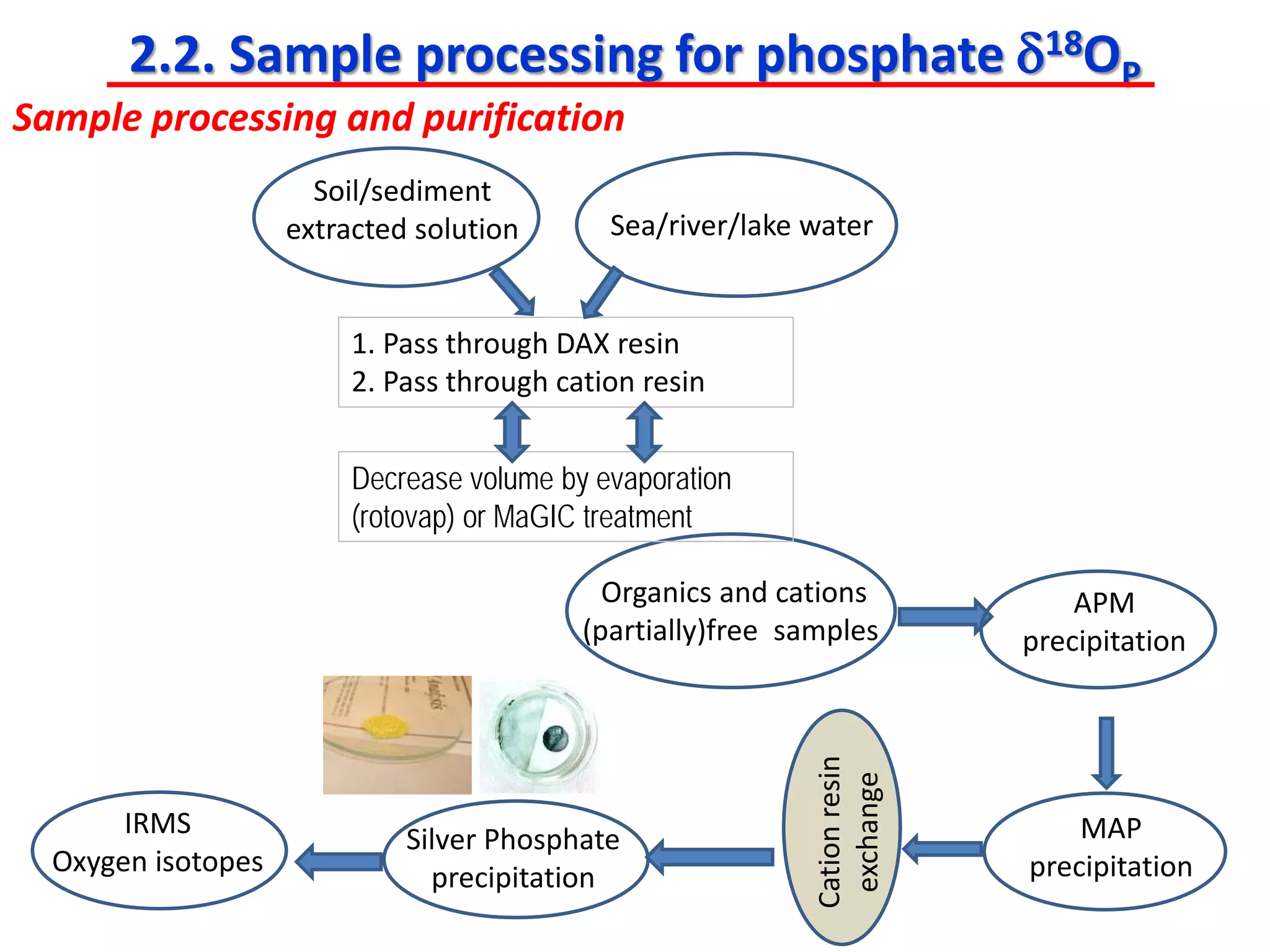
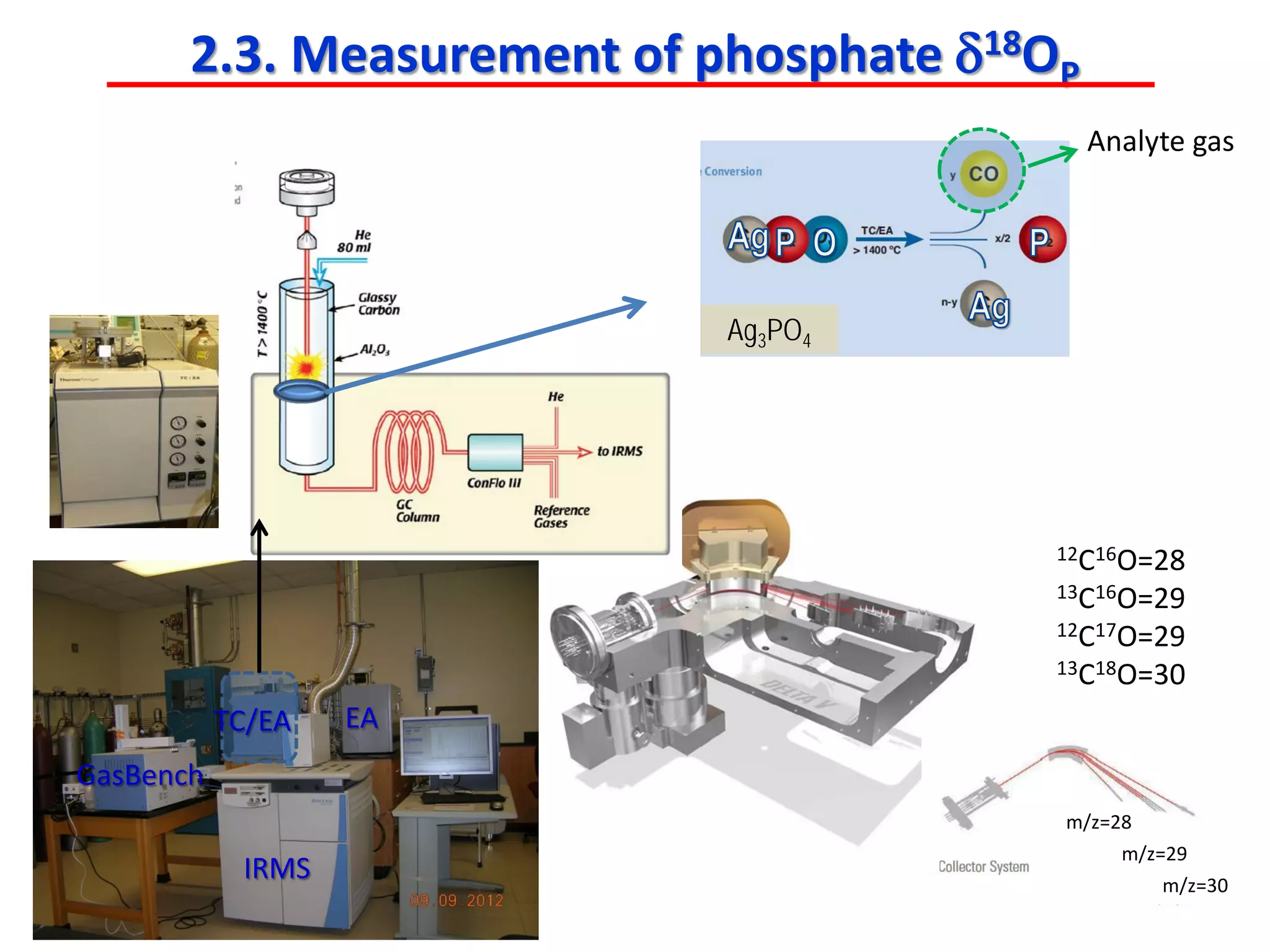
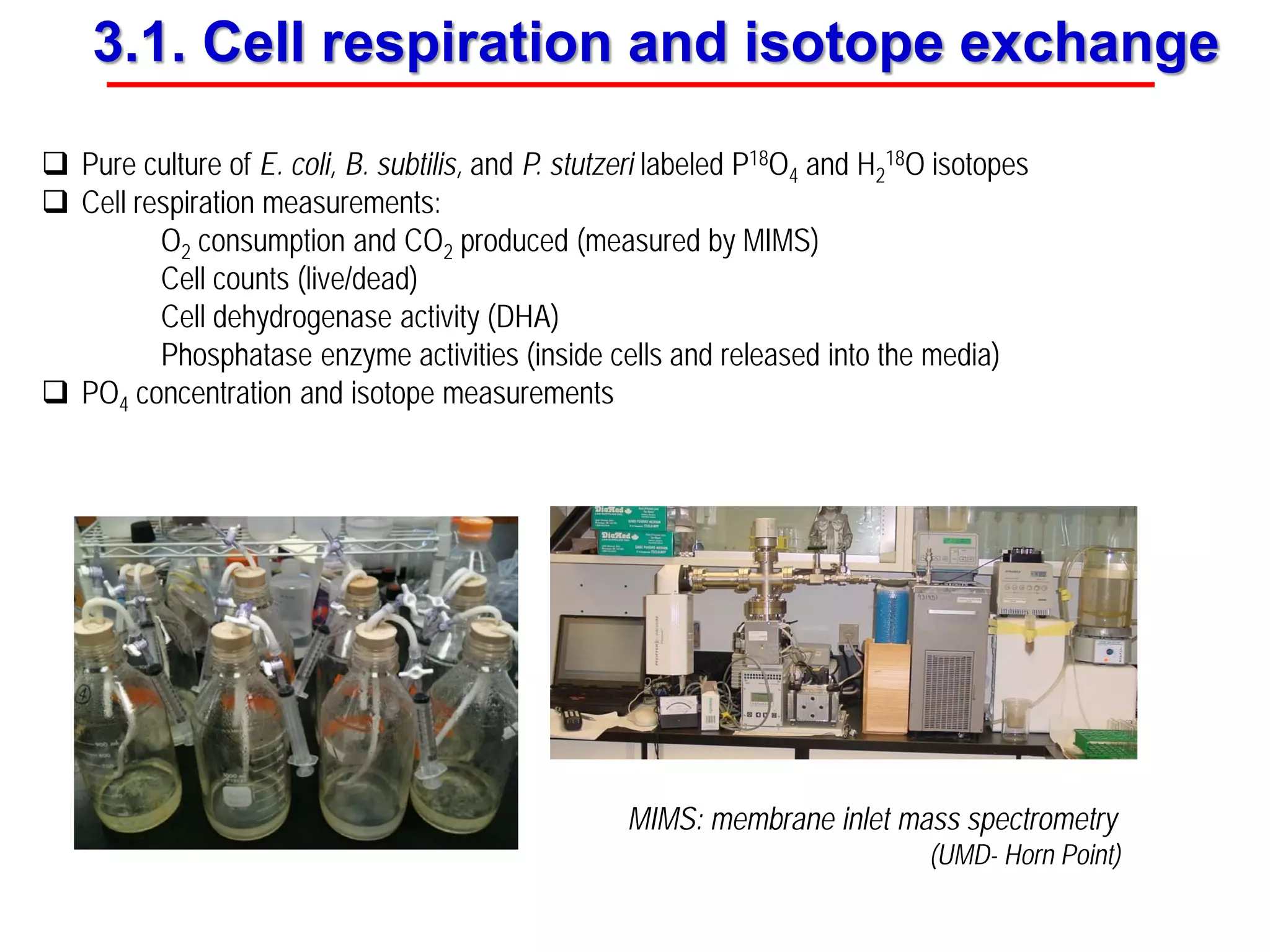
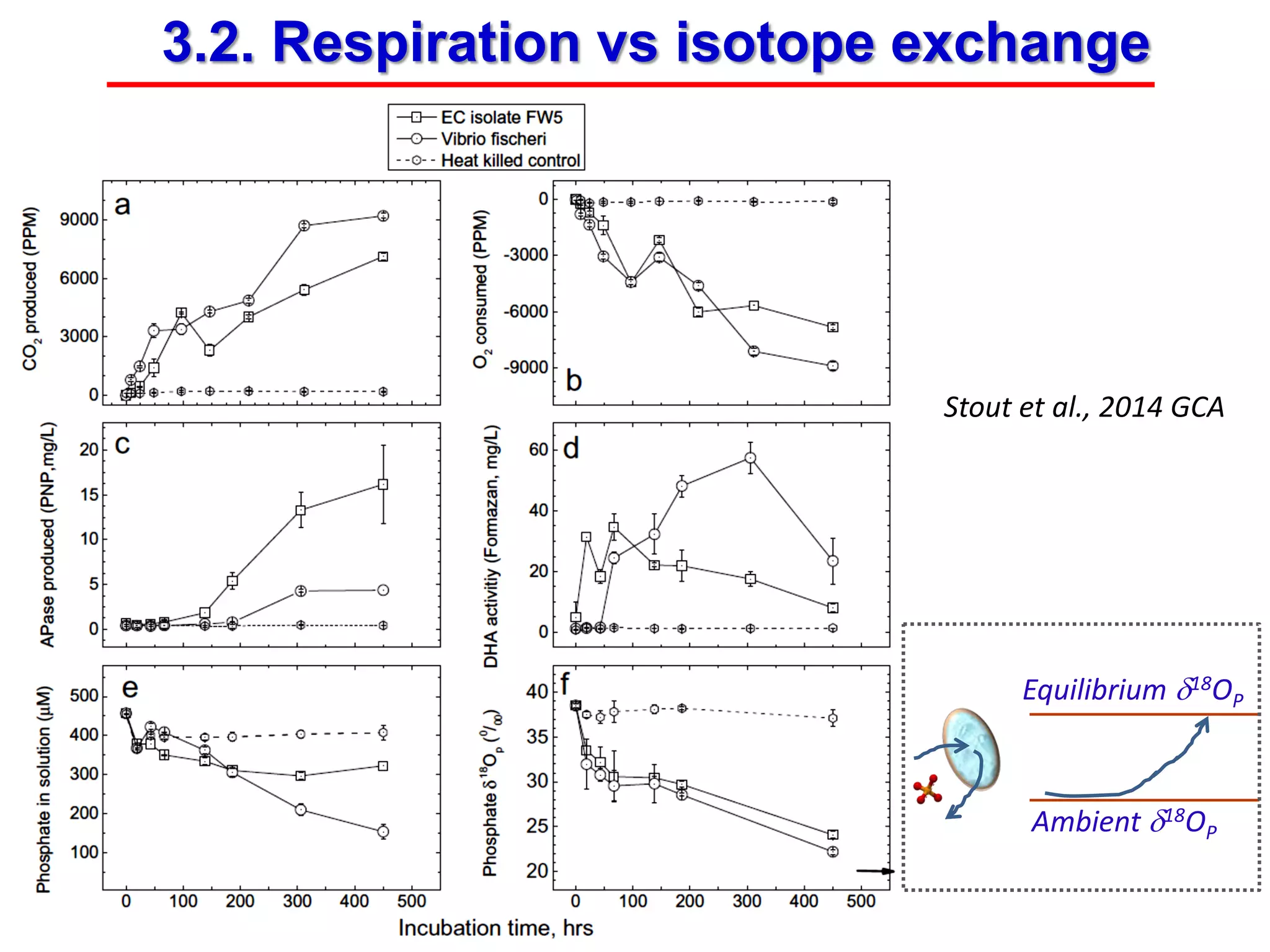
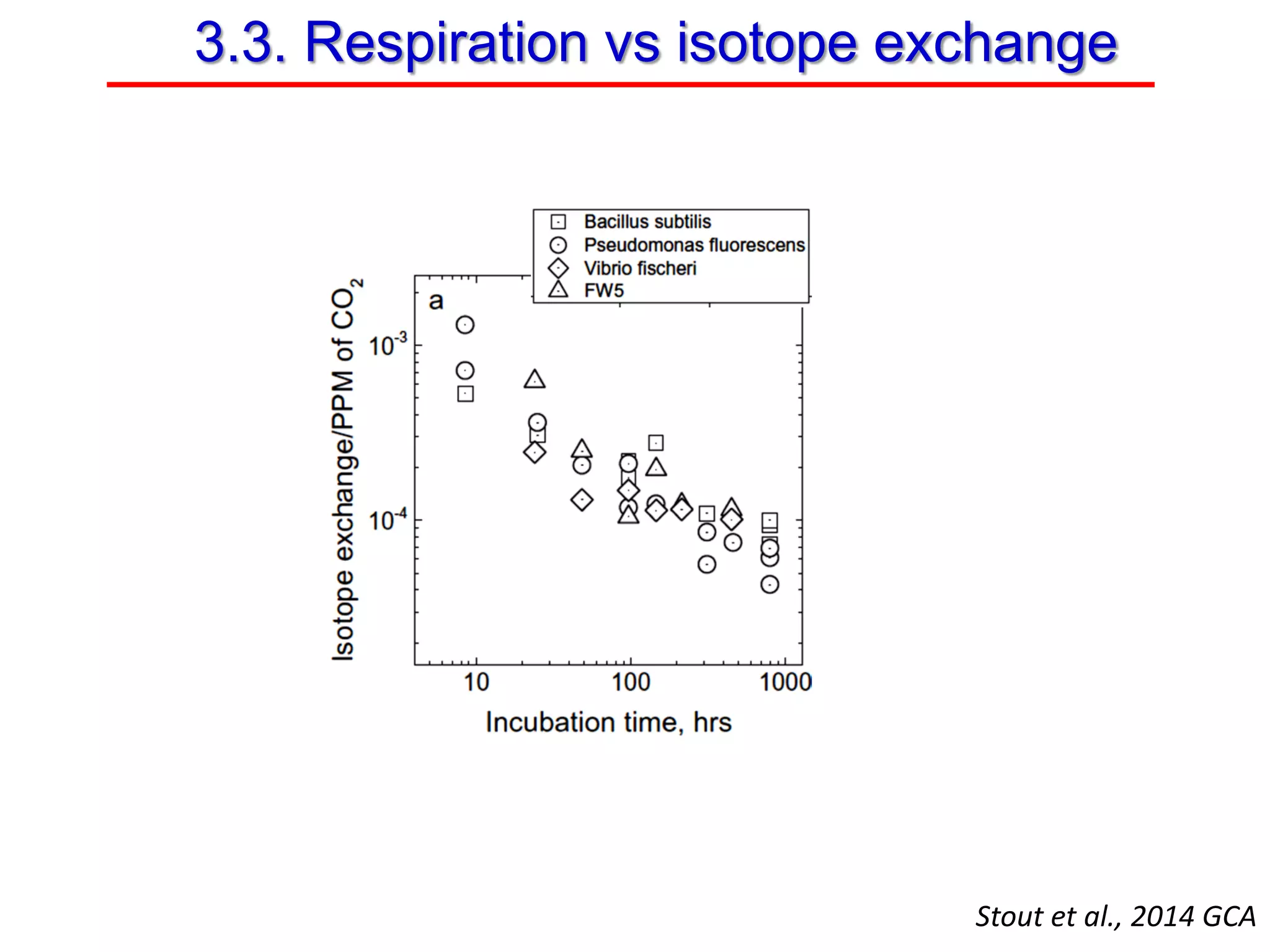
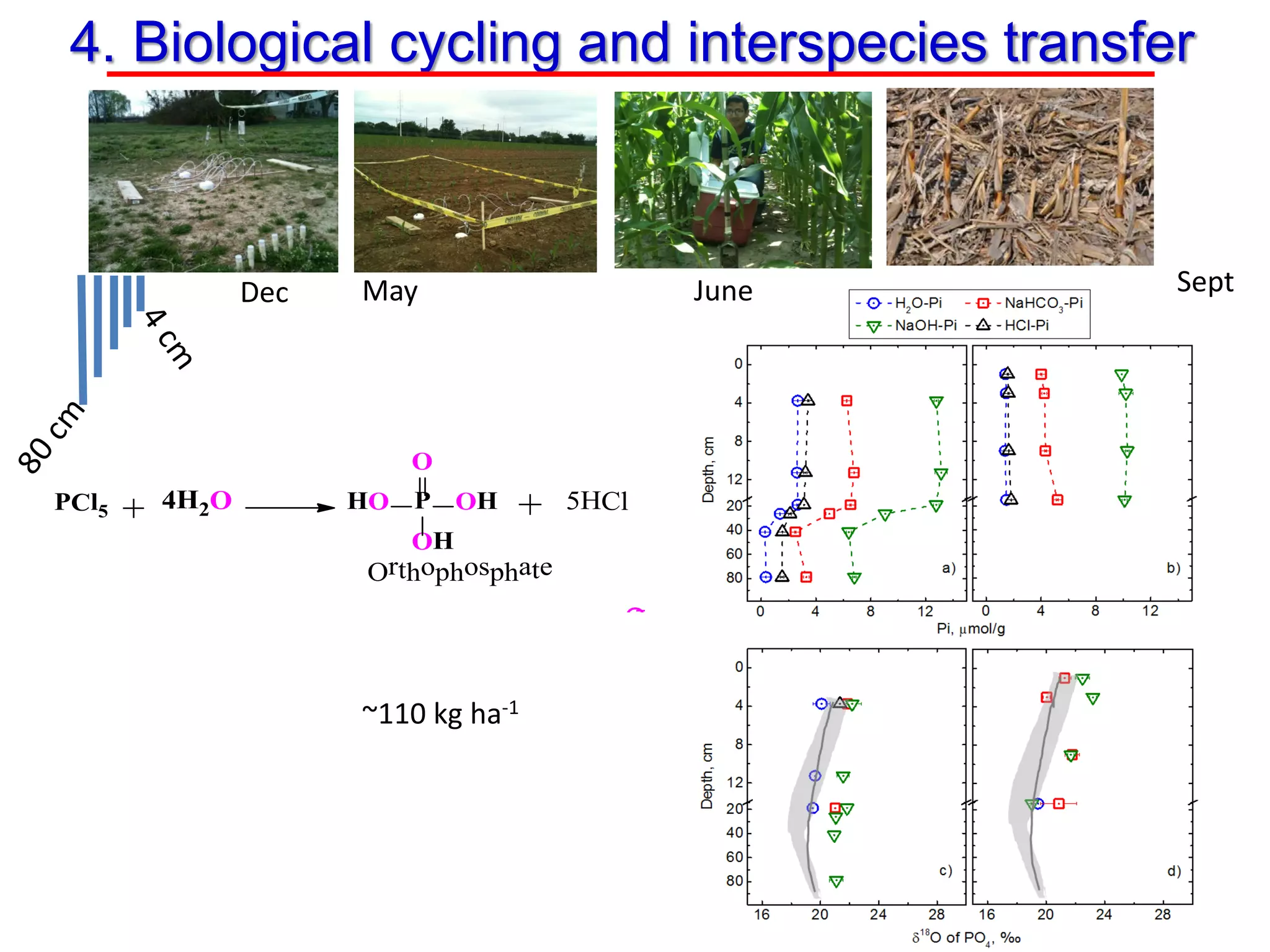
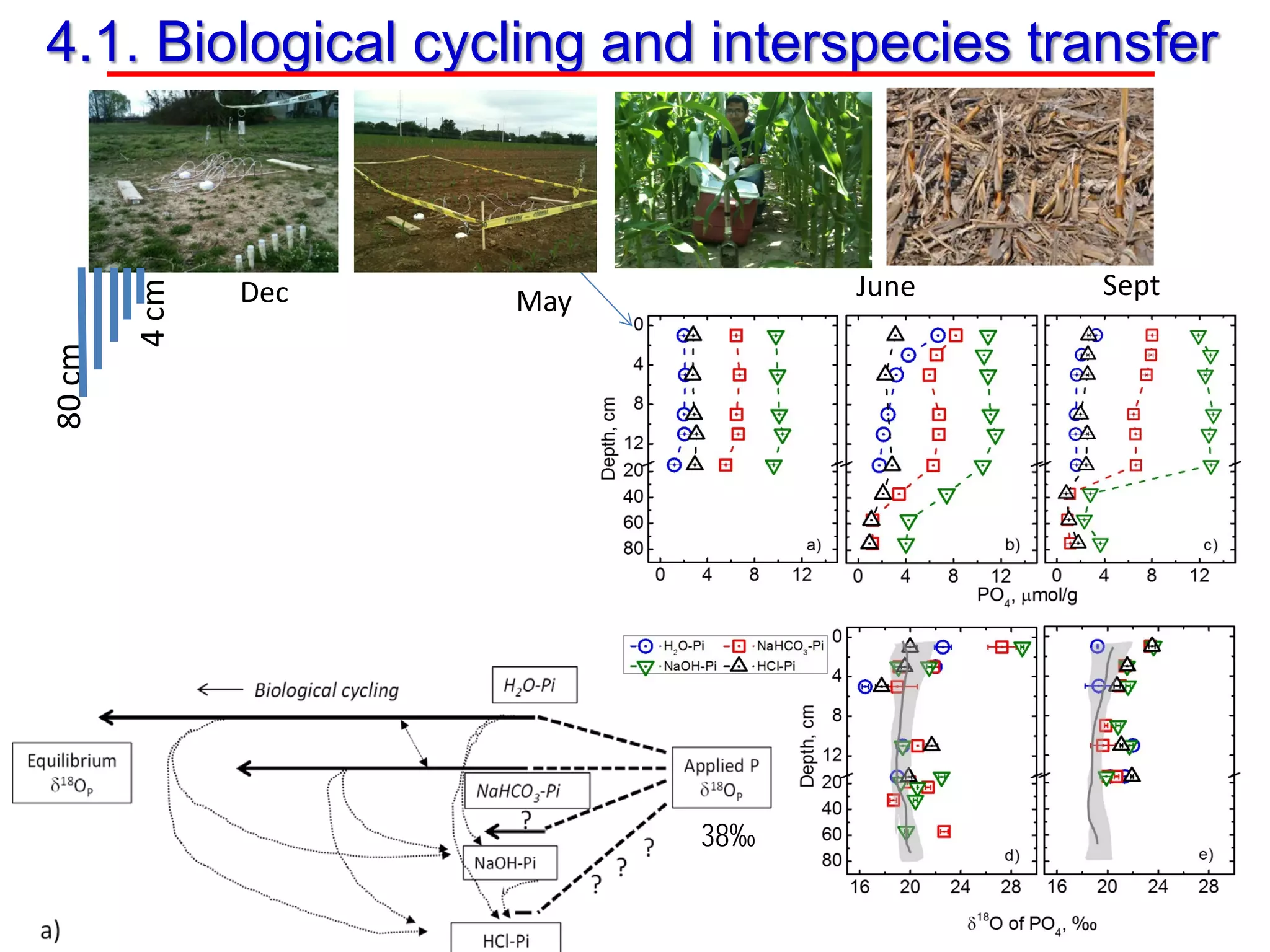
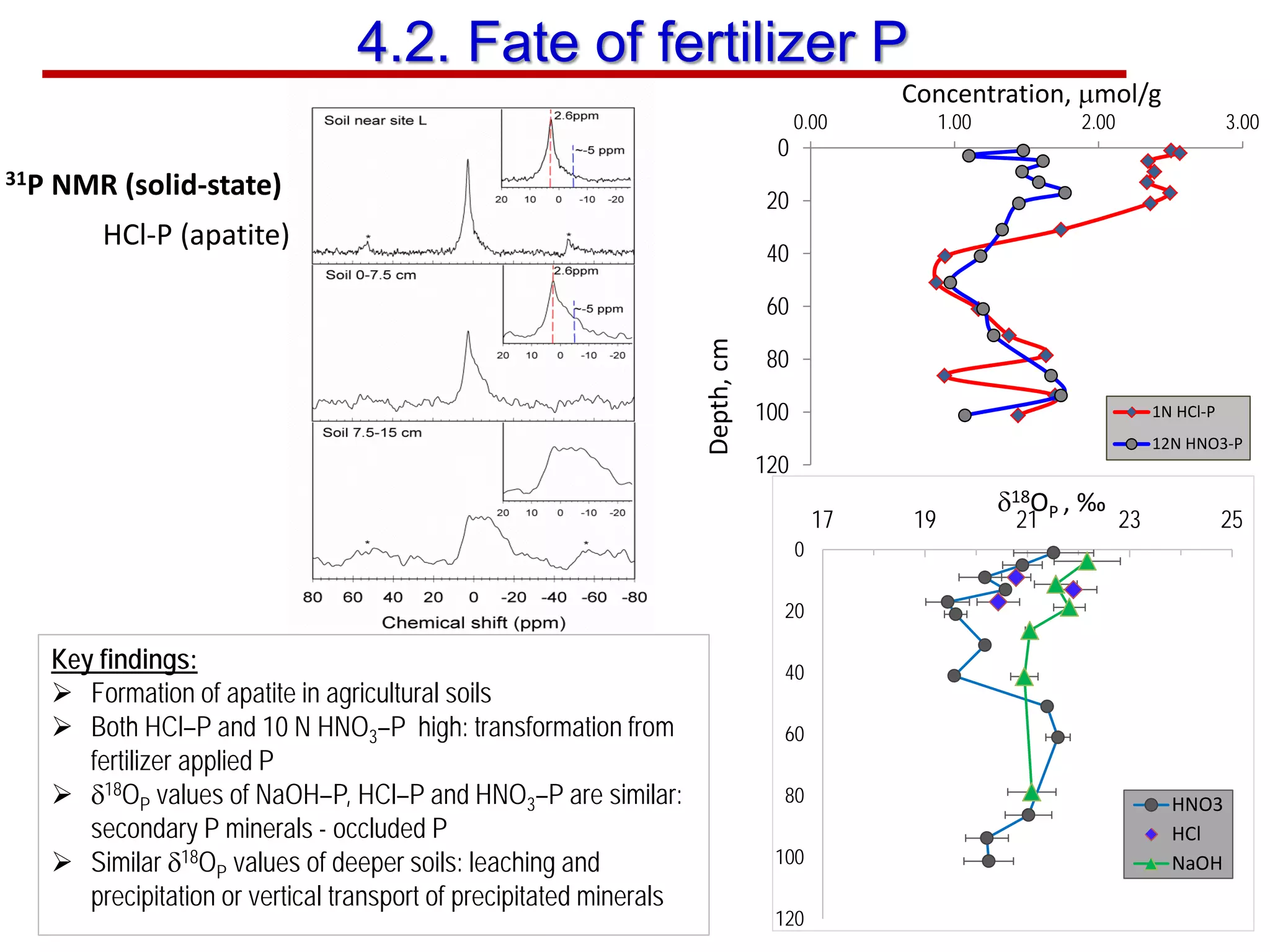
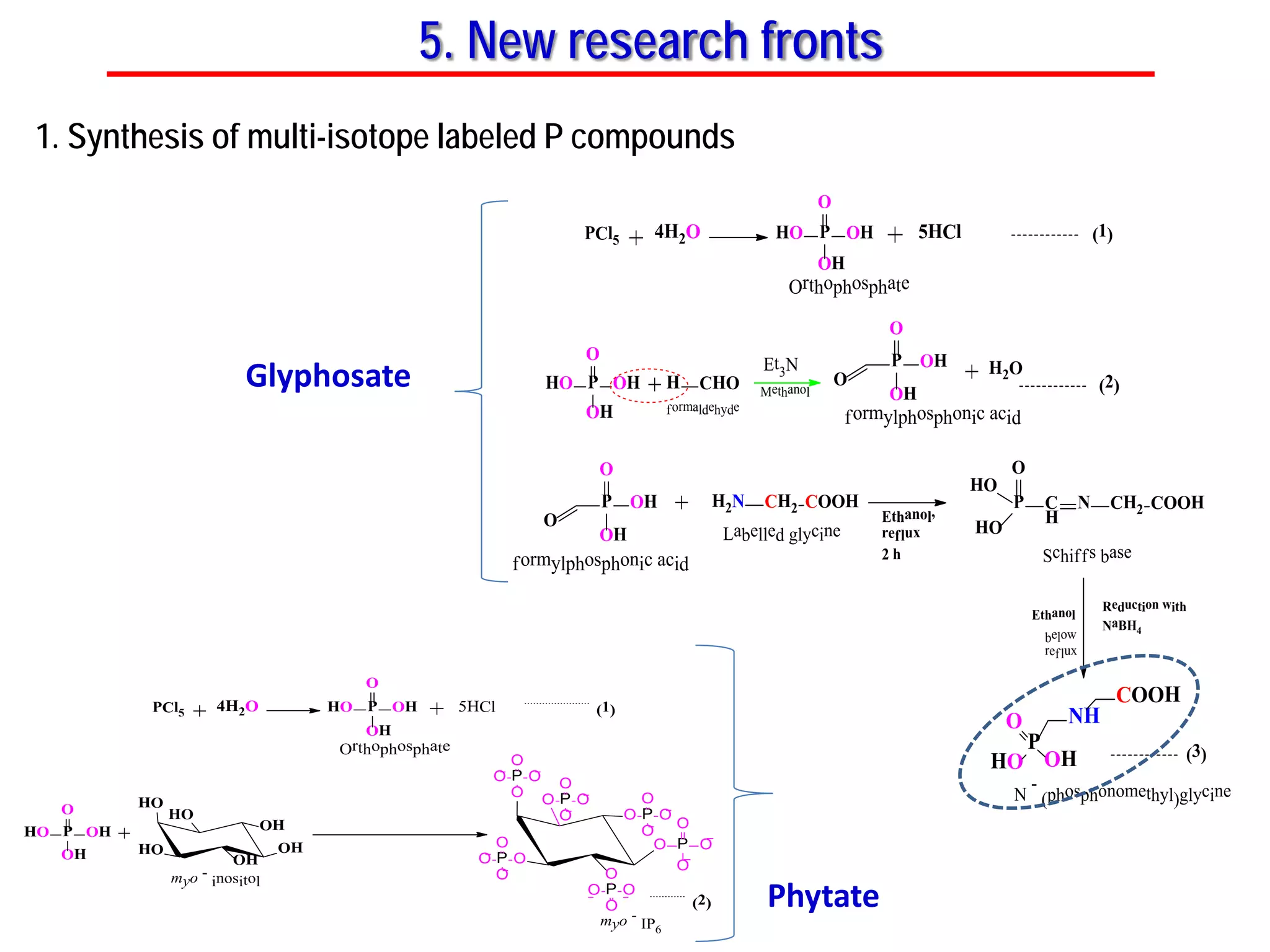
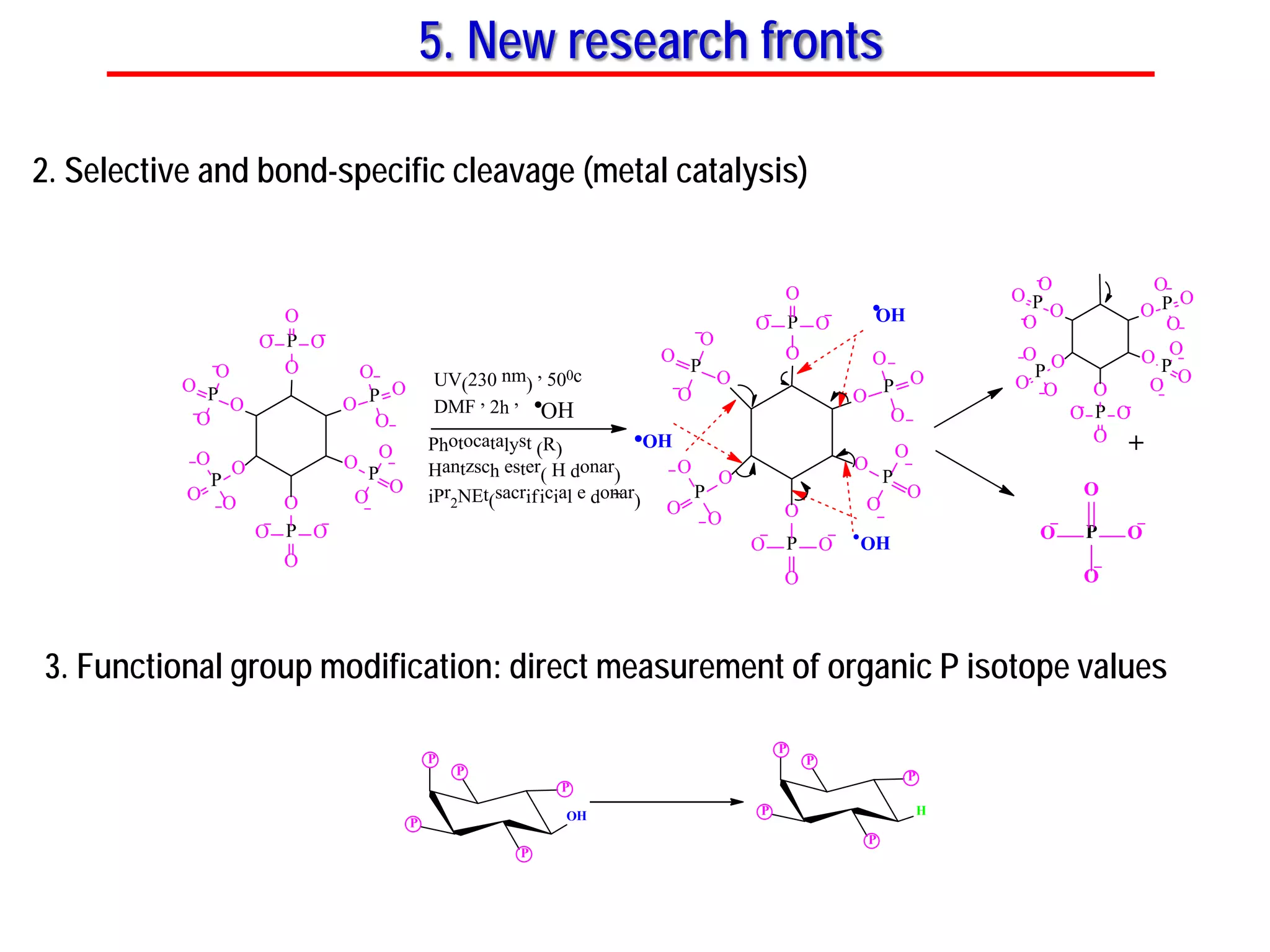
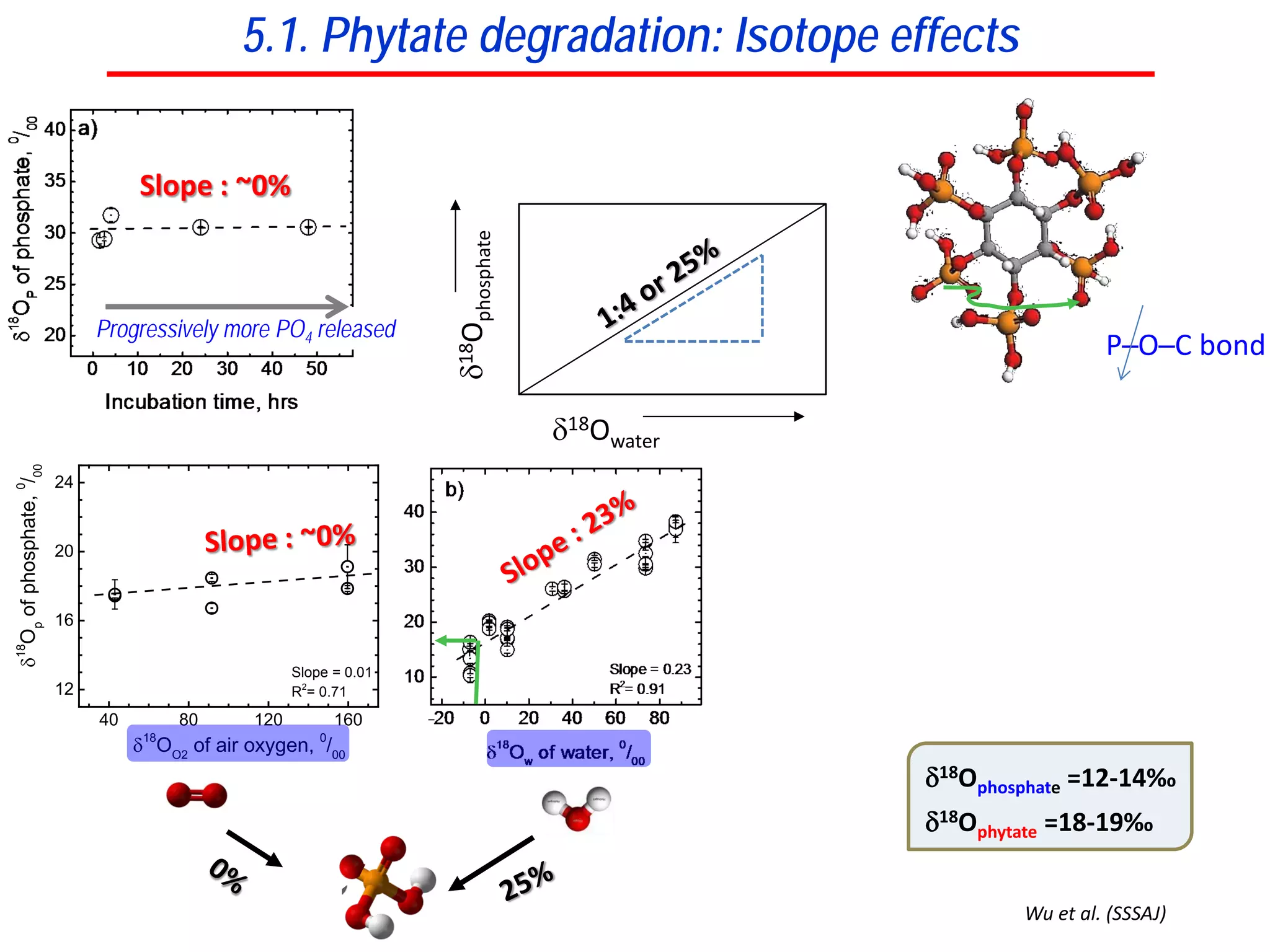
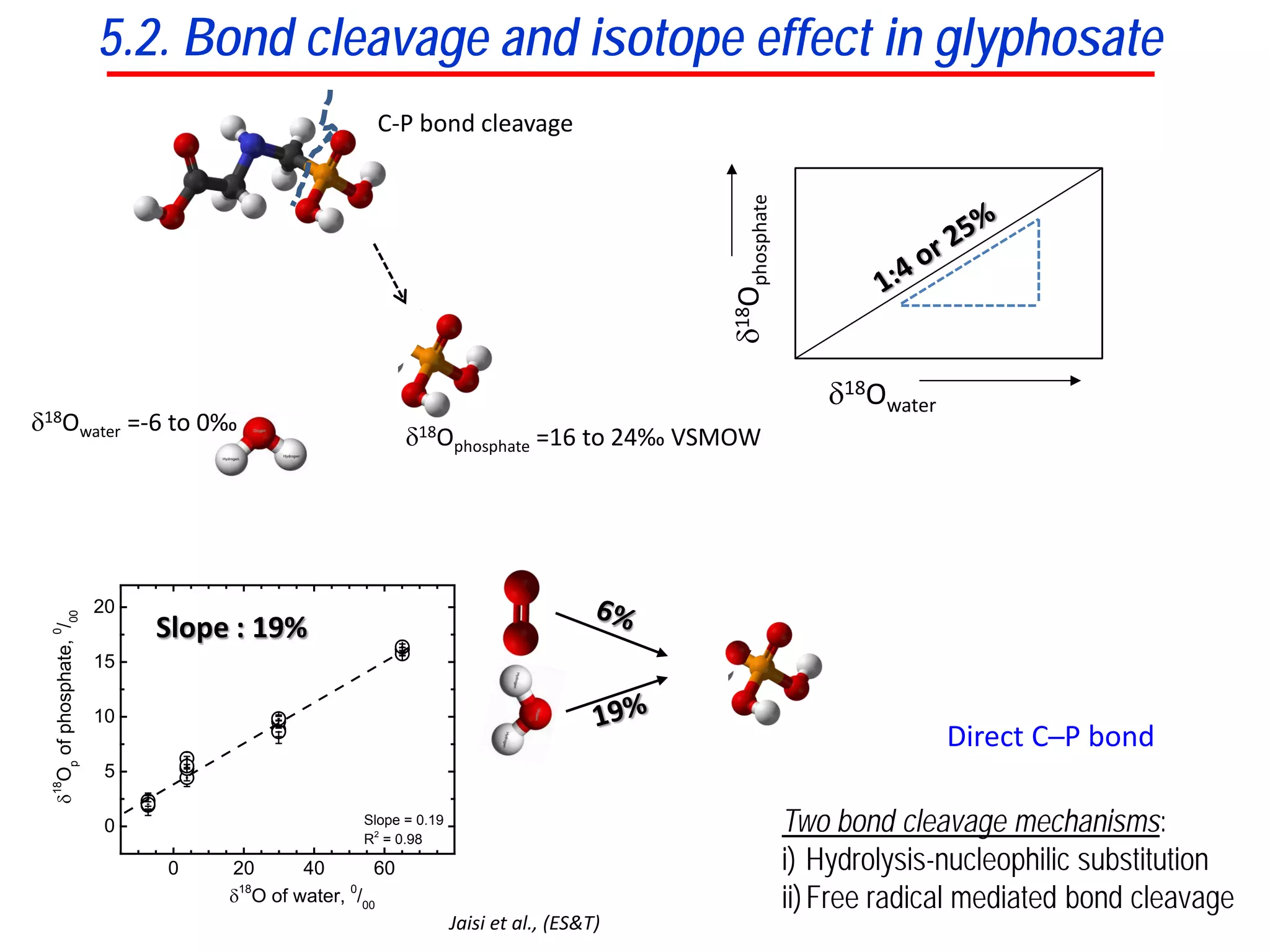
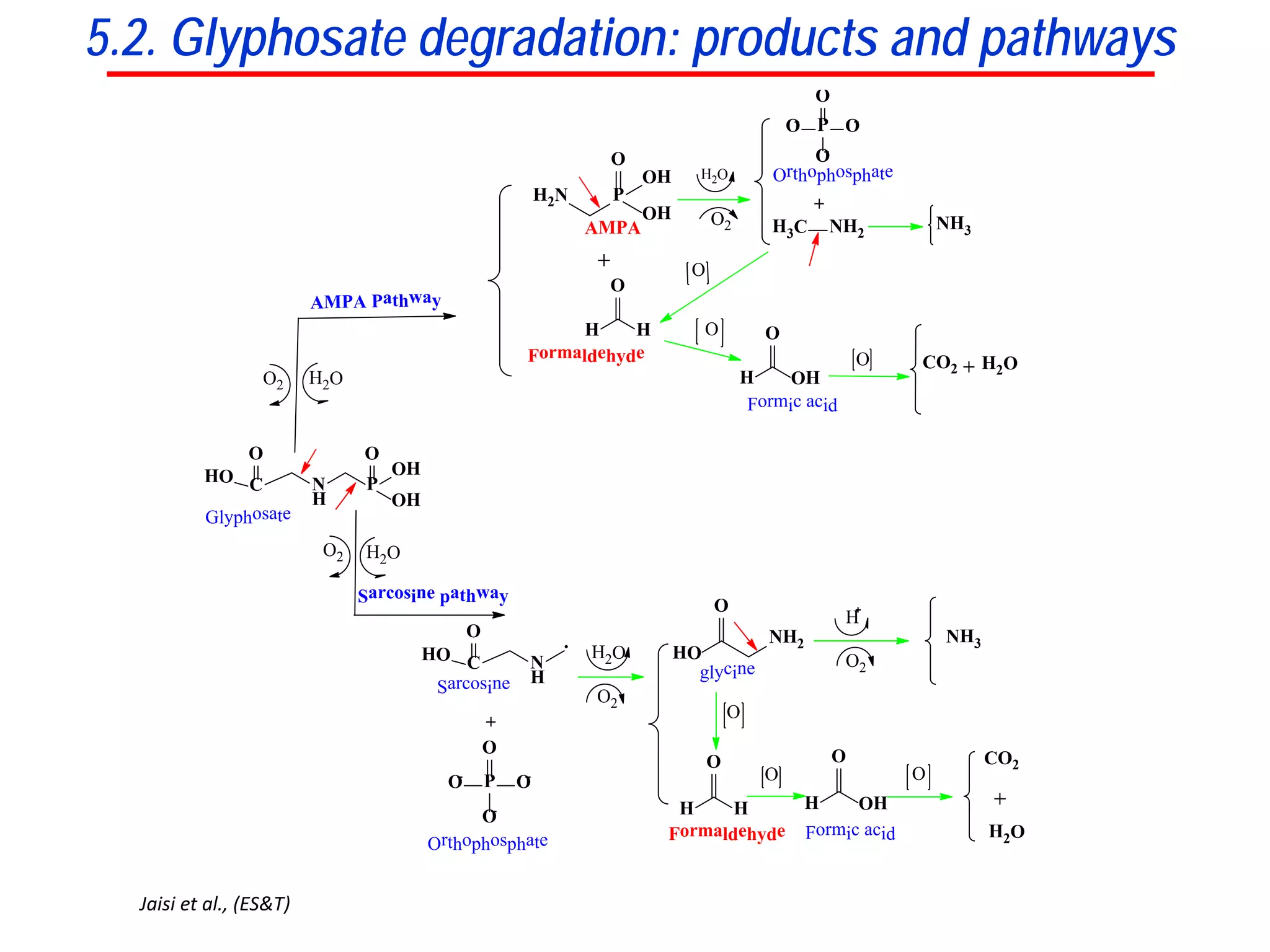
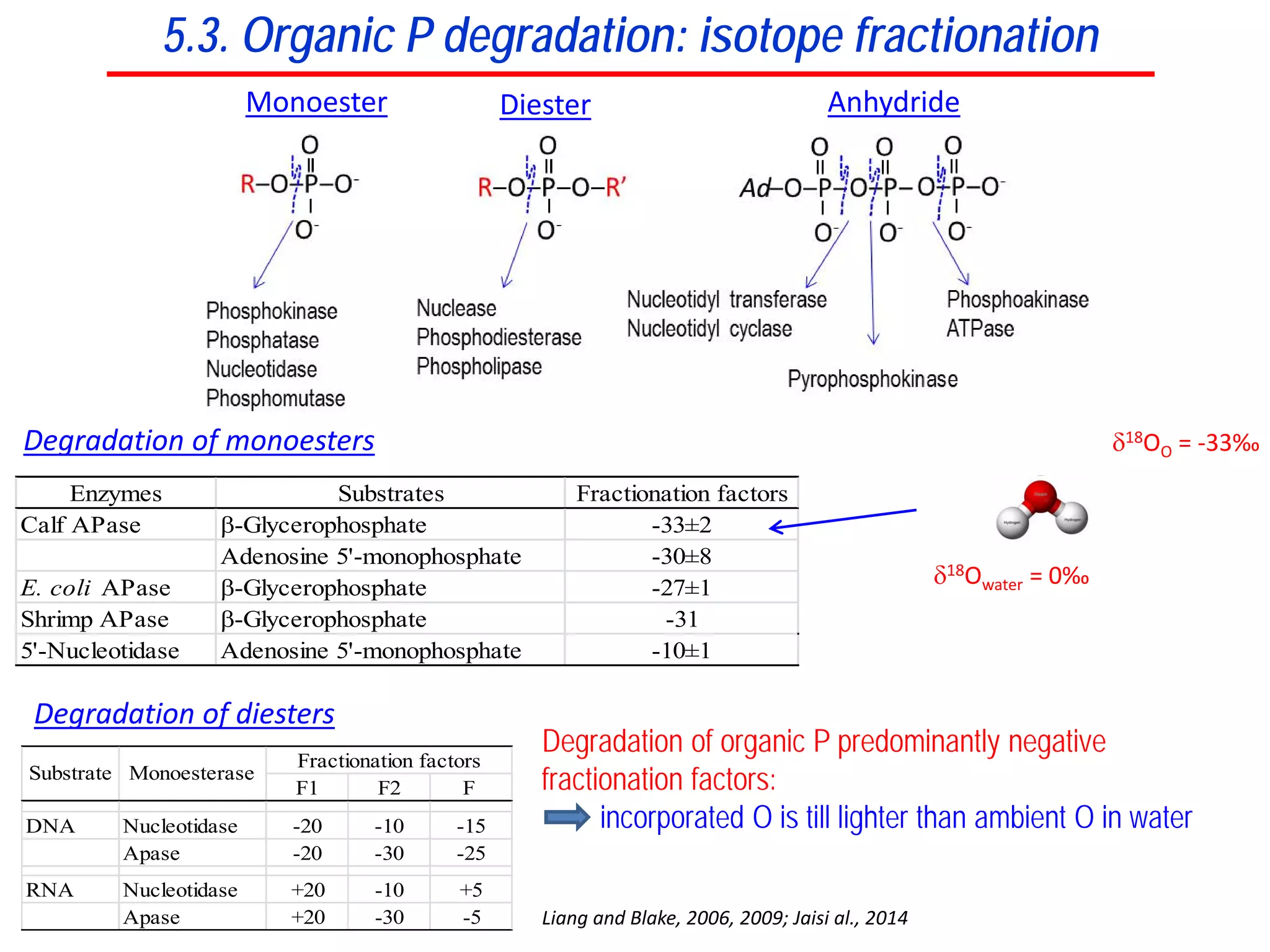
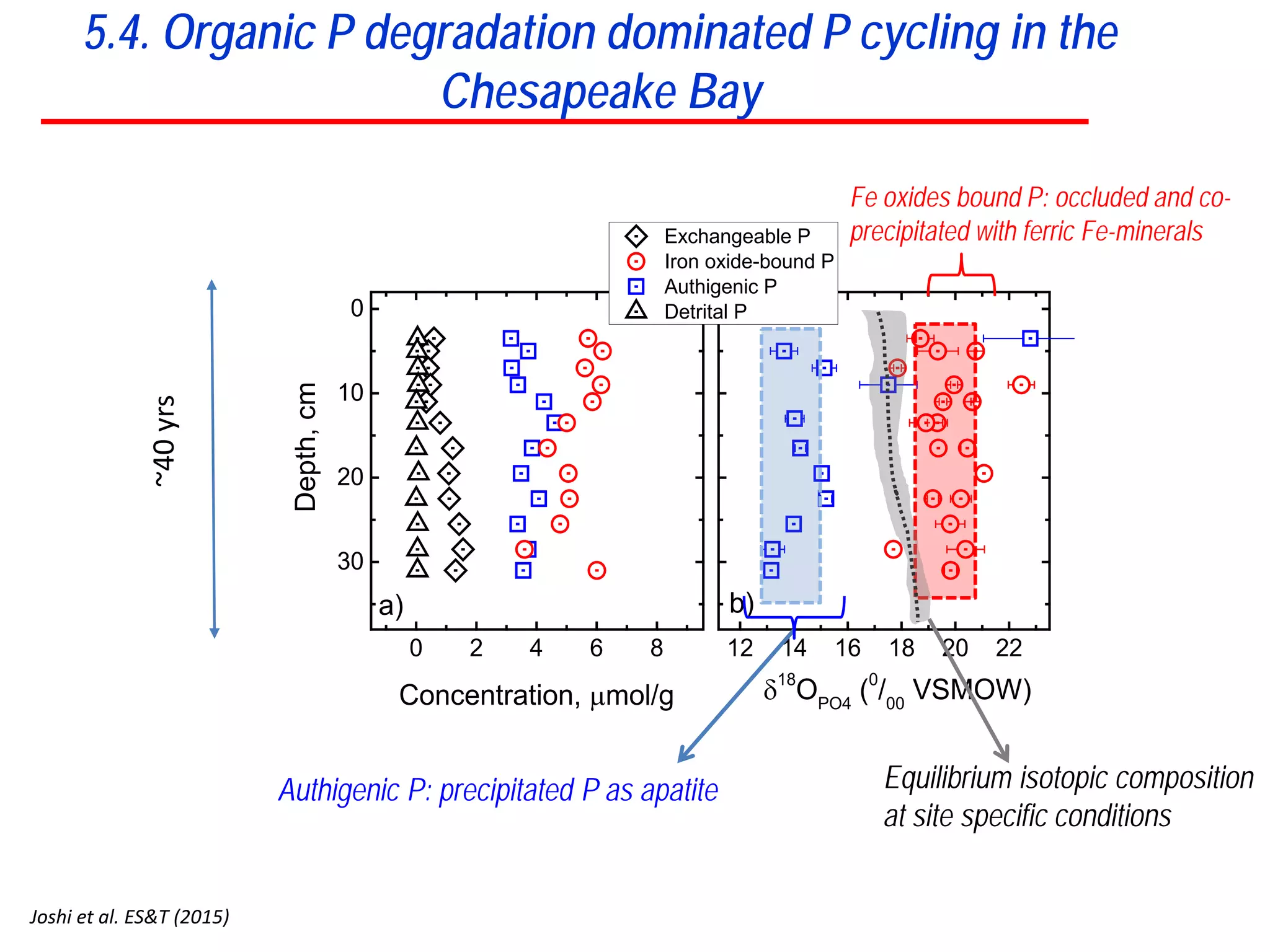
This document discusses using phosphate oxygen isotope ratios (δ18OP) to better understand phosphorus cycling in agricultural soils. It presents the goals of developing δ18OP as a tracer to identify the bioavailable P fraction in soils and track the long-term fate of externally applied P. It describes sample processing methods, measurement techniques, and initial findings showing transformation of fertilizer P into recalcitrant apatite P pools in agricultural soils. The document concludes that stable isotope labeling and tracking allows a deeper understanding of P sources, transfer, and transformations in natural environments.