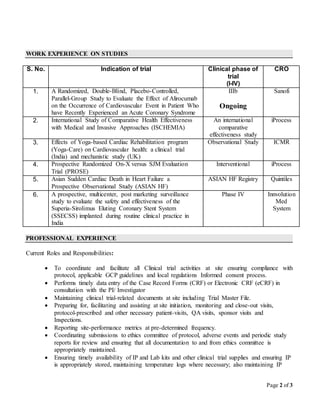
Anuj Kumar is a clinical research professional currently working as a Site Solutions Executive. He has over 5 years of experience working as a Clinical Research Coordinator on various clinical trials. His experience includes coordinating trial activities, ensuring protocol compliance, informed consent processes, data entry, maintaining documentation, and supporting site monitoring visits. He holds an Advanced Diploma in Clinical Research and degrees in Microbiology and Science. He is proficient in computer programs for clinical research and has participated in various clinical research training programs and workshops.