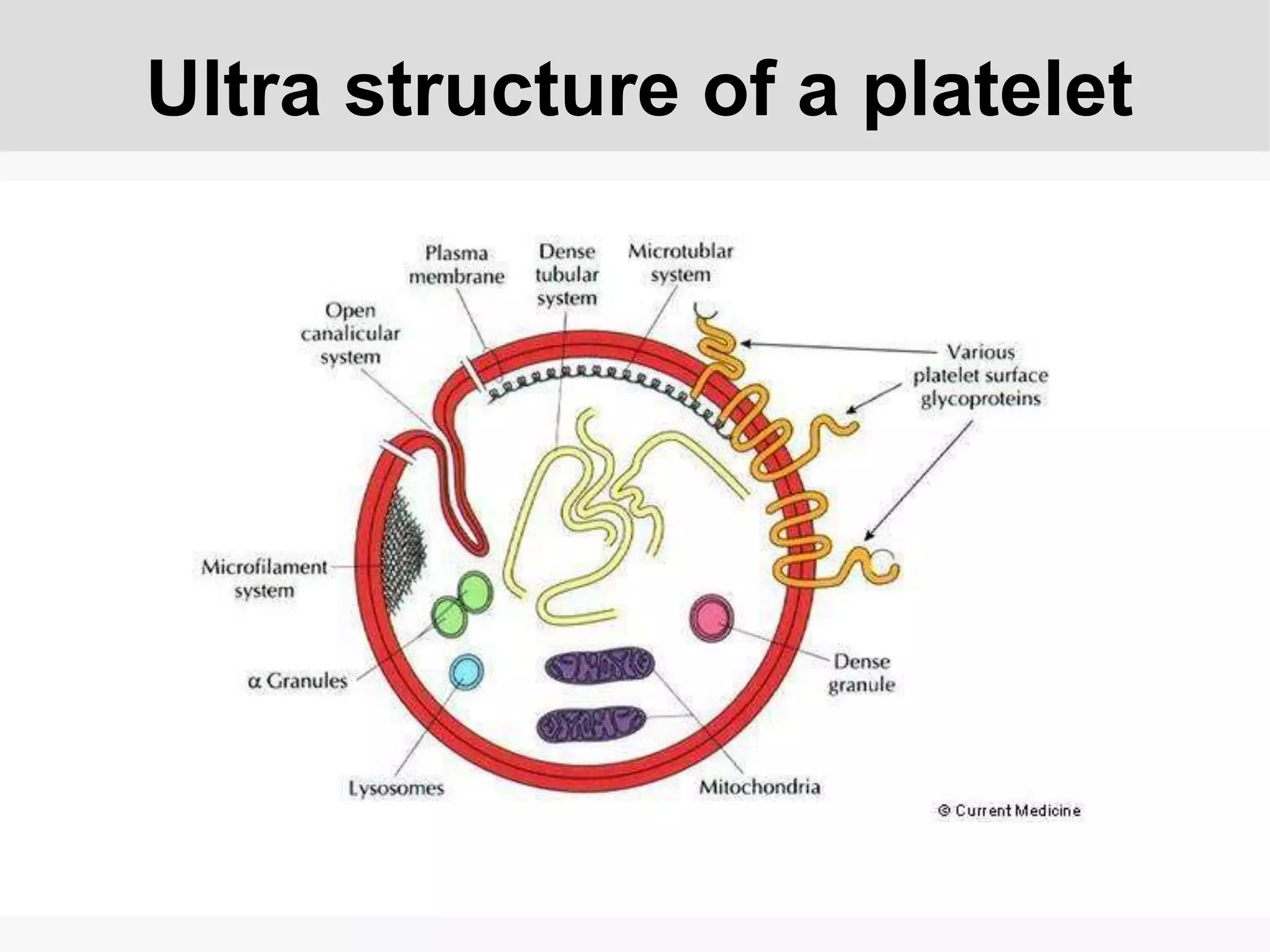
Platelets play an important role in hemostasis and thrombosis. Antiplatelet drugs like aspirin and clopidogrel are commonly used to prevent heart attacks and strokes. Aspirin works by irreversibly inhibiting the COX-1 enzyme in platelets to block thromboxane A2 production. Clopidogrel is a prodrug that irreversibly inhibits the P2Y12 receptor on platelets. Clinical trials show dual antiplatelet therapy with aspirin and clopidogrel reduces cardiovascular events in conditions like acute coronary syndrome compared to aspirin alone, though it increases bleeding risk. Newer P2Y12 inhibitors like prasugrel and ticagrelor are more potent but also associated with















































![Adverse Effects
• Bleeding (most common)
• Nausea, dyspepsis, diarrhea
• severe neutropenia (absolute neutrophil
count [ANC] <1500/mL
• Thrombopenia
• Thrombotic thrombocytopenic purpura](https://image.slidesharecdn.com/antiplatelets-170511154448/75/Antiplatelets-50-2048.jpg)



















































