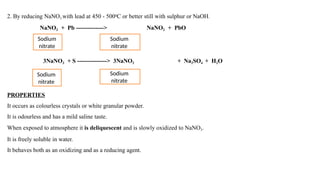
The document details the properties, methods of preparation, and uses of sodium nitrite (NaNO2) and sodium thiosulphate (Na2S2O3·5H2O). Sodium nitrite serves as an antidote for cyanide poisoning and is synthesized through various reduction methods, while sodium thiosulphate, also used for cyanide poisoning, is made by reacting sodium sulfide with sulfur dioxide. Both compounds are deliquescent, soluble in water, and undergo changes in state at elevated temperatures.