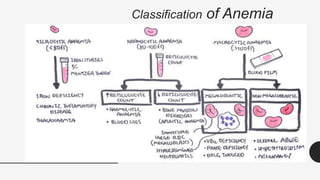
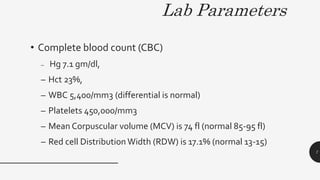
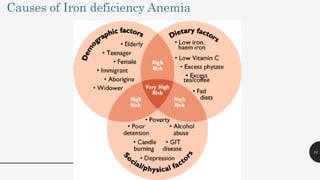
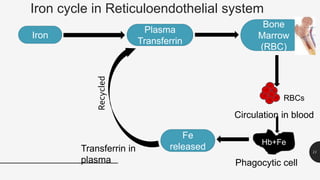
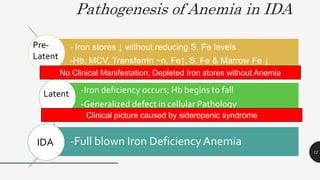
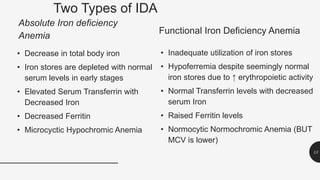
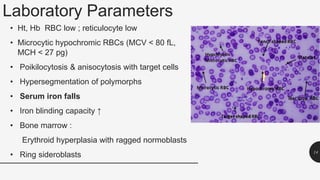
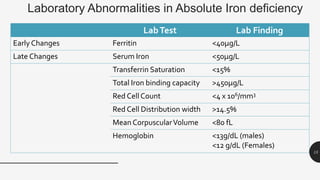
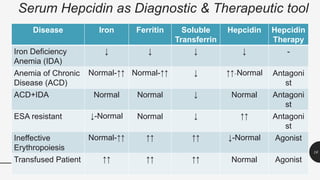
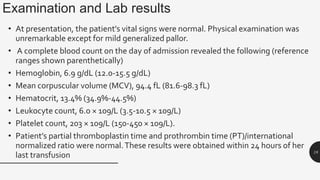
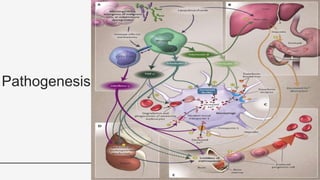
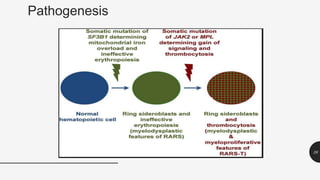
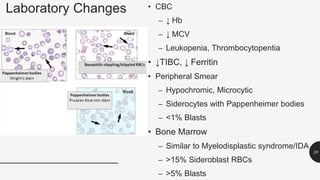
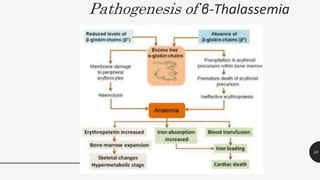
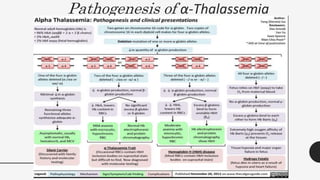
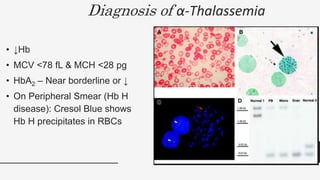
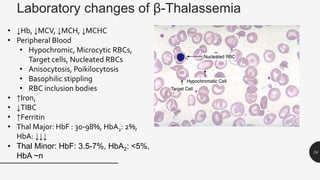
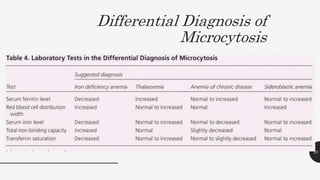
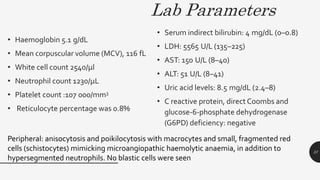
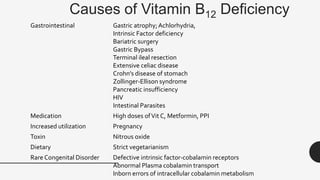
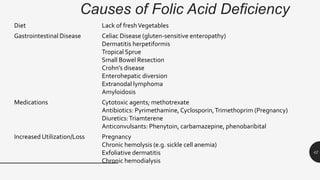
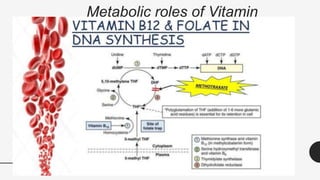
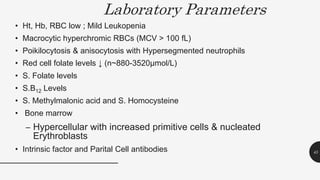
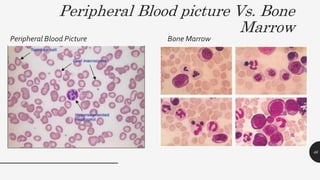
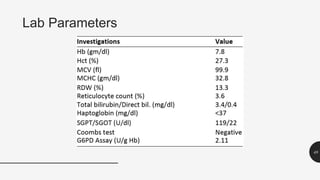
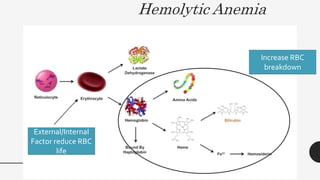
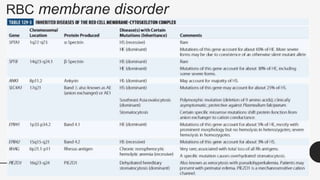
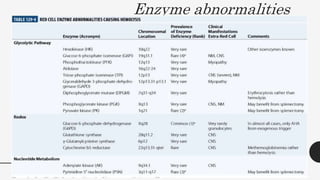
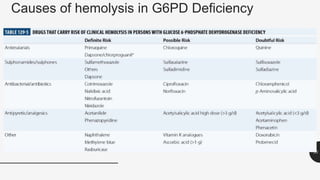
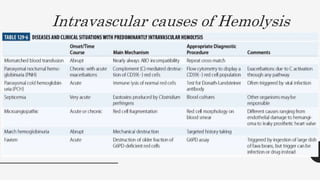
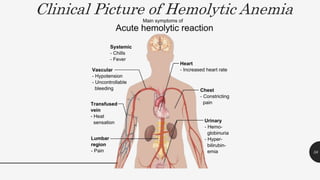
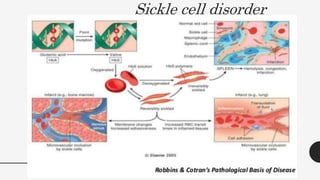
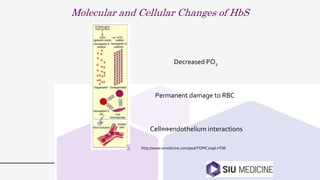
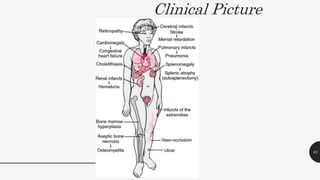
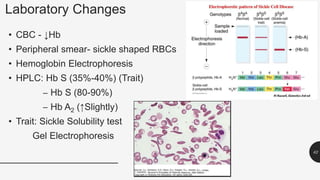
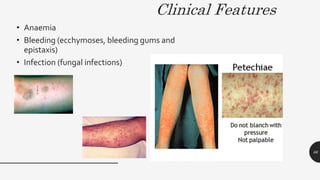
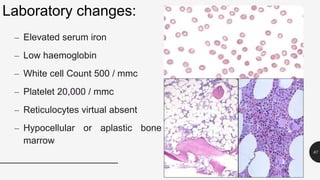
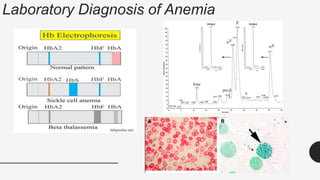
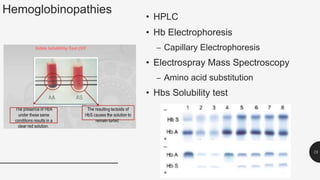
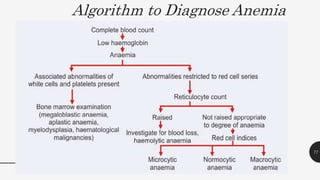
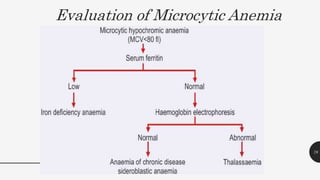
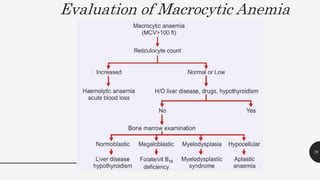
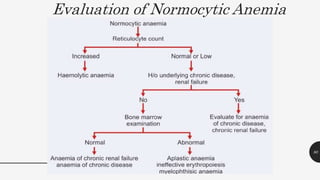
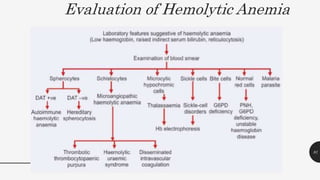
This document provides an overview of anemia, including its definition, types, causes, diagnosis, and laboratory findings. It discusses the main types of anemia - microcytic (iron deficiency, thalassemia), macrocytic (vitamin B12 and folate deficiency), and hemolytic. For each type, it describes the pathogenesis, clinical presentation, and characteristic lab abnormalities to aid in diagnosis. Case studies are also presented to demonstrate how the lab results can help classify the underlying cause of anemia in different patients.