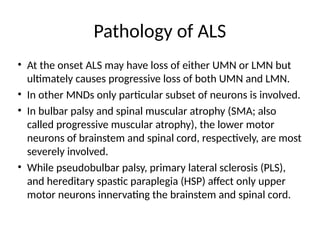
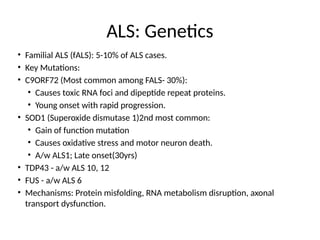
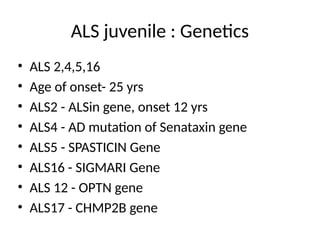
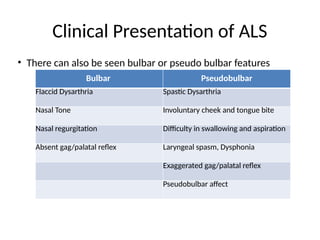
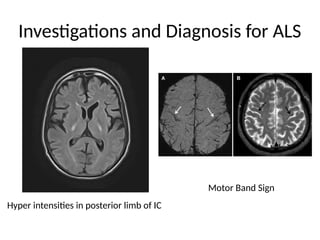
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease characterized by the degeneration of upper and lower motor neurons, leading to muscle atrophy and other severe symptoms. It can be classified into sporadic (90%) and familial (10%) types, with both genetic and environmental factors contributing to its onset and progression. Current treatments include riluzole and edaravone, with emerging therapies like gene therapy and stem cell treatments under exploration, but prognosis remains poor with an average survival of 3-5 years post-diagnosis.