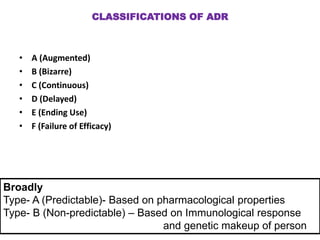
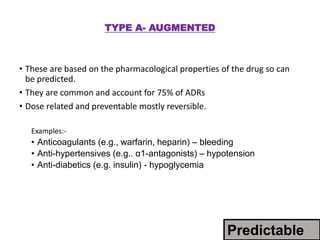
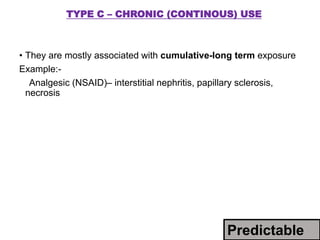
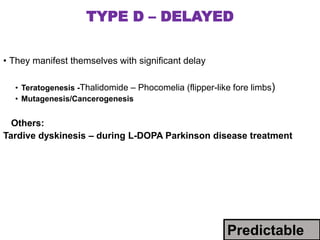
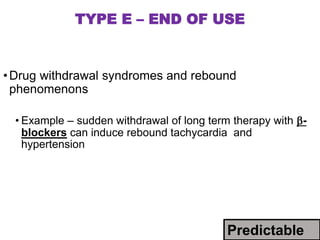
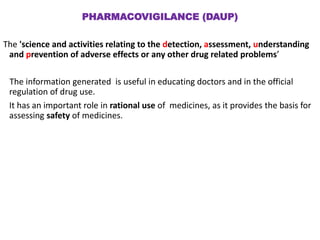
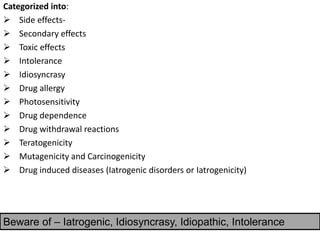
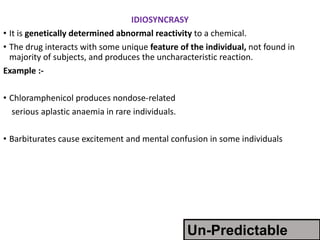
This document discusses adverse drug reactions and pharmacovigilance. It defines an adverse drug reaction as an unwanted change caused by a drug at normal doses that requires treatment or a dose decrease. Adverse drug events are untoward occurrences during treatment that are not necessarily caused by the treatment. The document classifies adverse drug reactions into types A, B, C, D and E based on factors like dose, time of onset, and mechanism. It also discusses preventing adverse drug effects through appropriate use and monitoring for new symptoms after starting treatment. Pharmacovigilance aims to detect, understand and prevent adverse drug reactions through postmarketing surveillance.