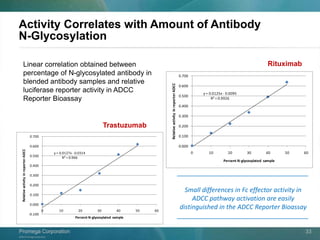
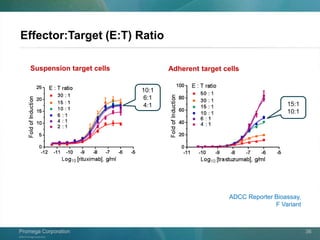
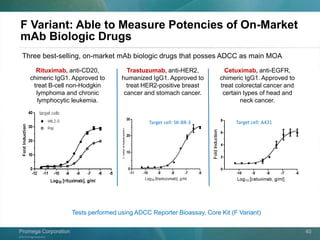
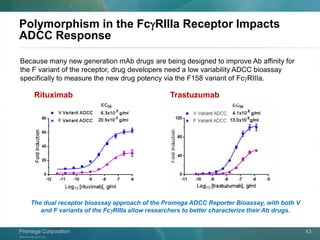
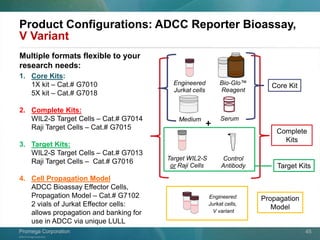
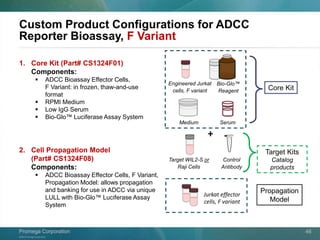
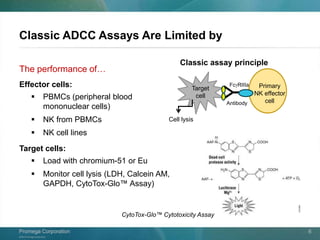
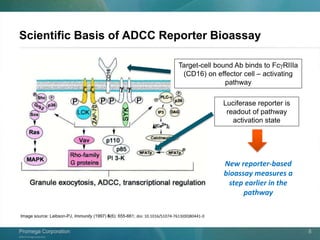
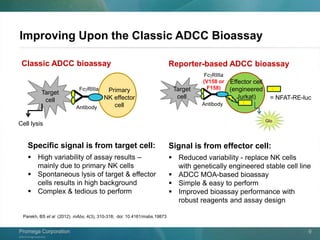
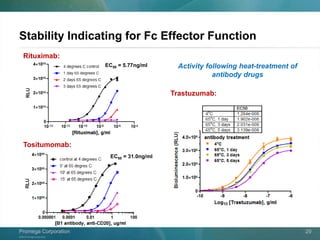
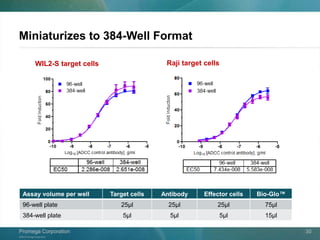
The document presents a detailed overview of the novel ADCC reporter bioassays developed by Promega Corporation, which improve upon traditional assays by enabling better quantification of antibody effector functions. These assays utilize engineered cell lines and a frozen, thaw-and-use format to reduce variability and enhance reproducibility in testing. The document outlines the advantages of these bioassays, including their stability, precision, and ability to assess the impact of Fc receptor polymorphisms on therapeutic outcomes.

![©2013 Promega Corporation.
Promega CorporationPromega Corporation 4
Characteristics of a Valid Bioassay Are…
-10 -9 -8 -7 -6 -5
0
5
10
15
20
25
30
35
plate1
plate2
plate3
plate4
Log10 [B1 antibody], g/ml
FoldofInduction
Repeatability
Design:
Two analysts
Three days
Four plates per day
100% vs 50%
100% vs 75%
100% vs 125%
100% vs 150% -10 -9 -8 -7 -6 -5
0
5
10
15
20
25
30
35
100%
50%
150%
Log10 [B1 antibody], g/ml
FoldofInduction
Y=1.026X-5.126
R2=0.995
Relative Potency
Linearity
Log [control antibody], g/ml
Log [control antibody], g/ml
Validation of Analytical
Procedures:
Accuracy
Precision:
Repeatability (intra-assay
precision)
Intermediate precision (day
to day, analyst to analyst)
Reproducibility (lab to lab)
Specificity
Linearity
Range
Robustness
- ICH Guideline Q2 [R1]](https://image.slidesharecdn.com/adccreporterbioassaymarch2014rev01-140313132458-phpapp02/85/ADCC-Reporter-Bioassay-V-and-F-Variants-Novel-Bioluminescent-Cell-Based-Assays-for-Quantifying-Fc-Effector-Function-of-Antibodies-4-320.jpg)



















![©2013 Promega Corporation.
Promega CorporationPromega Corporation 28
ADCC Reporter Bioassay is Robust
-10 -9 -8 -7 -6 -5
0
10
20
30
1
6
7
14
Log10 [B1 antibody], g/ml
FoldofInduction
-10 -9 -8 -7 -6 -5
0
10
20
30
1
1
7
13
16
Log10 [B1 antibody], g/ml
FoldofInduction
Time of induction
E:T ratio and cell # per well
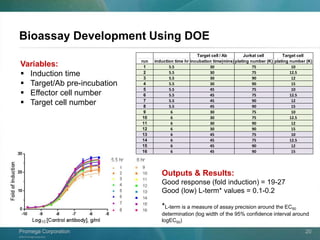
Run Induction time EC50
1 6.0hr 3.15x10-8 g/ml
2 5.5hr 3.83x10-8 g/ml
Run E:T ratio E cell # T cell # EC50
1 7.5:1 75k 10k 3.09x10-8 g/ml
2 6:1 90k 15k 3.83x10-8 g/ml](https://image.slidesharecdn.com/adccreporterbioassaymarch2014rev01-140313132458-phpapp02/85/ADCC-Reporter-Bioassay-V-and-F-Variants-Novel-Bioluminescent-Cell-Based-Assays-for-Quantifying-Fc-Effector-Function-of-Antibodies-28-320.jpg)

![©2013 Promega Corporation.
Promega CorporationPromega Corporation 32
Deglycosylated Herceptin
-12 -10 -8 -6 -4
0
5100 5
1100 6
2100 6
unt
10%unt/ 90% degly
EC50
unt
2.037e-008
10%unt/ 90% degly
7.174e-008
log [ab], (g/ml)
RLU
Deglycosylated Herceptin
-12 -10 -8 -6 -4
0
5100 5
1100 6
2100 6
unt
100% degly
EC50
unt
1.988e-008
100% degly
3.202e-008
log [ab], (g/ml)
RLU
Deglycosylated Herceptin
-12 -10 -8 -6 -4
0
5100 5
1100 6
2100 6
EC50
unt
1.720e-008
20%unt/80% degly
4.626e-008
unt
20%unt/80% degly
log [ab], (g/ml)
RLU
Deglycosylated Herceptin
-12 -10 -8 -6 -4
0
5100 5
1100 6
2100 6
unt
30%unt/ 70% degly
EC50
unt
2.002e-008
30%unt/ 70% degly
4.153e-008
log [ab], (g/ml)
RLU
Deglycosylated Herceptin
-12 -10 -8 -6 -4
0
5100 5
1100 6
2100 6
unt
40%unt/ 60% degly
EC50
unt
2.082e-008
40%unt/ 60% degly
3.486e-008
log [ab], (g/ml)RLU
Deglycosylated Herceptin
-12 -10 -8 -6 -4
0
5100 5
1100 6
2100 6
unt
50%unt/ 50% degly
EC50
unt
2.110e-008
50%unt/ 50% degly
2.990e-008
log [ab], (g/ml)
RLU
Target cells: SKBR3; Unt = 100% glycosylated
Sensitive to Detect Differences in Glycosylation](https://image.slidesharecdn.com/adccreporterbioassaymarch2014rev01-140313132458-phpapp02/85/ADCC-Reporter-Bioassay-V-and-F-Variants-Novel-Bioluminescent-Cell-Based-Assays-for-Quantifying-Fc-Effector-Function-of-Antibodies-32-320.jpg)