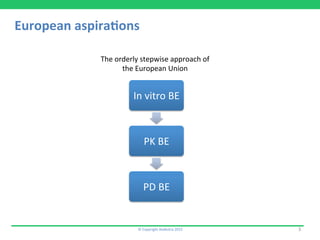
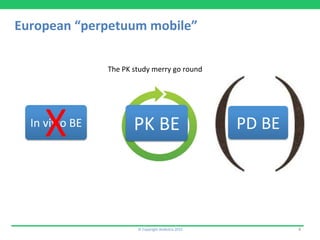
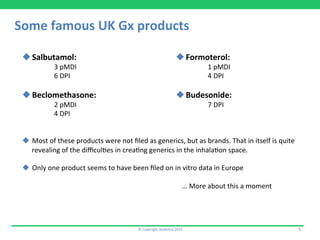
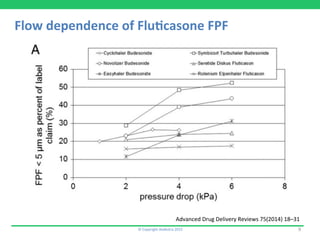
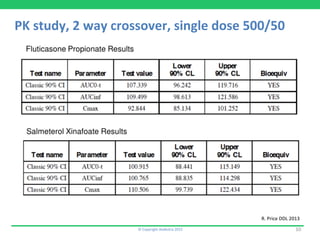
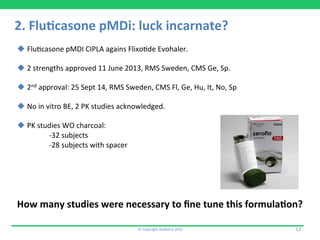
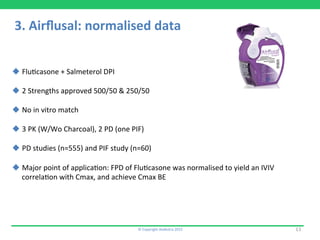
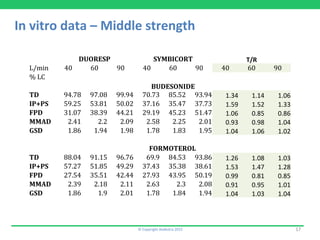
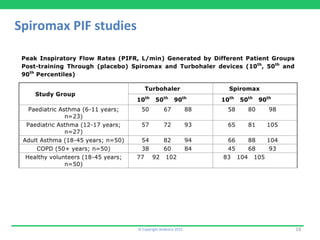
This document discusses the challenges of obtaining approval for generic inhaled drug products in Europe. It provides examples of several inhaled generics that have received approval and the types of data submitted, including:
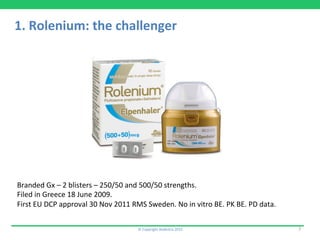
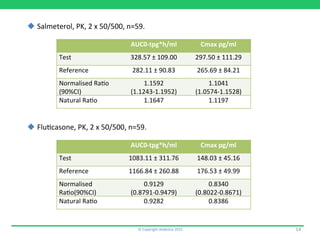
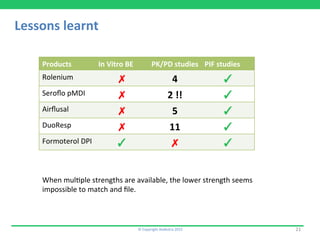
1) Rolenium, a salmeterol/fluticasone combination, which received approval after submitting pharmacokinetic and pharmacodynamic data but no in vitro bioequivalence data.
2) A fluticasone propionate MDI from Cipla that was approved with two pharmacokinetic studies but no in vitro data.
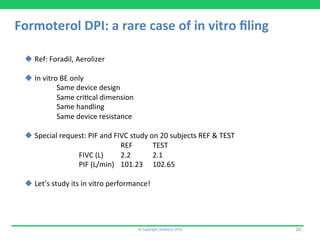
3) Airflusal, a salmeterol/fluticasone combination DPI that was approved with normalized pharmacokinetic data but no demonstration of