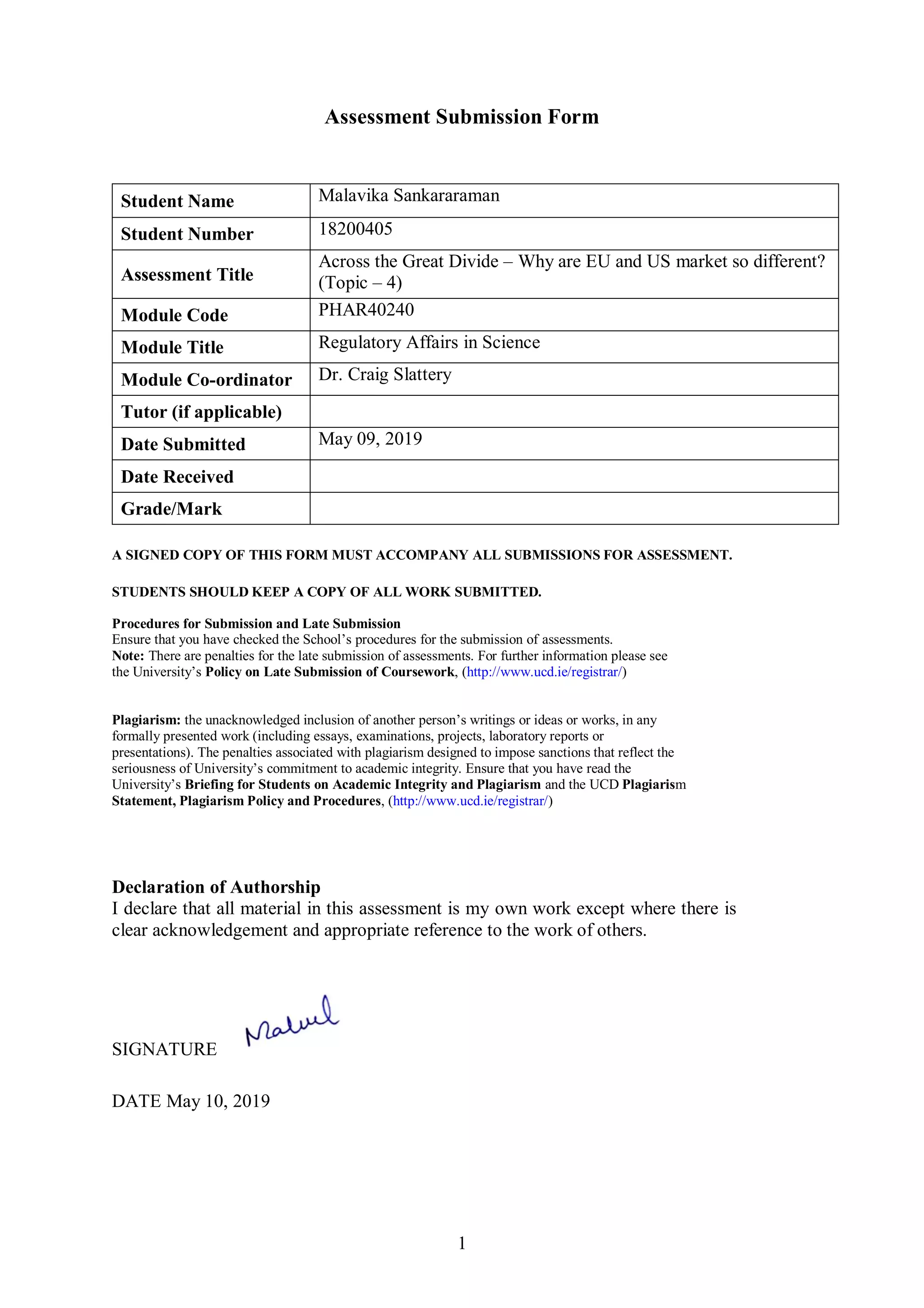
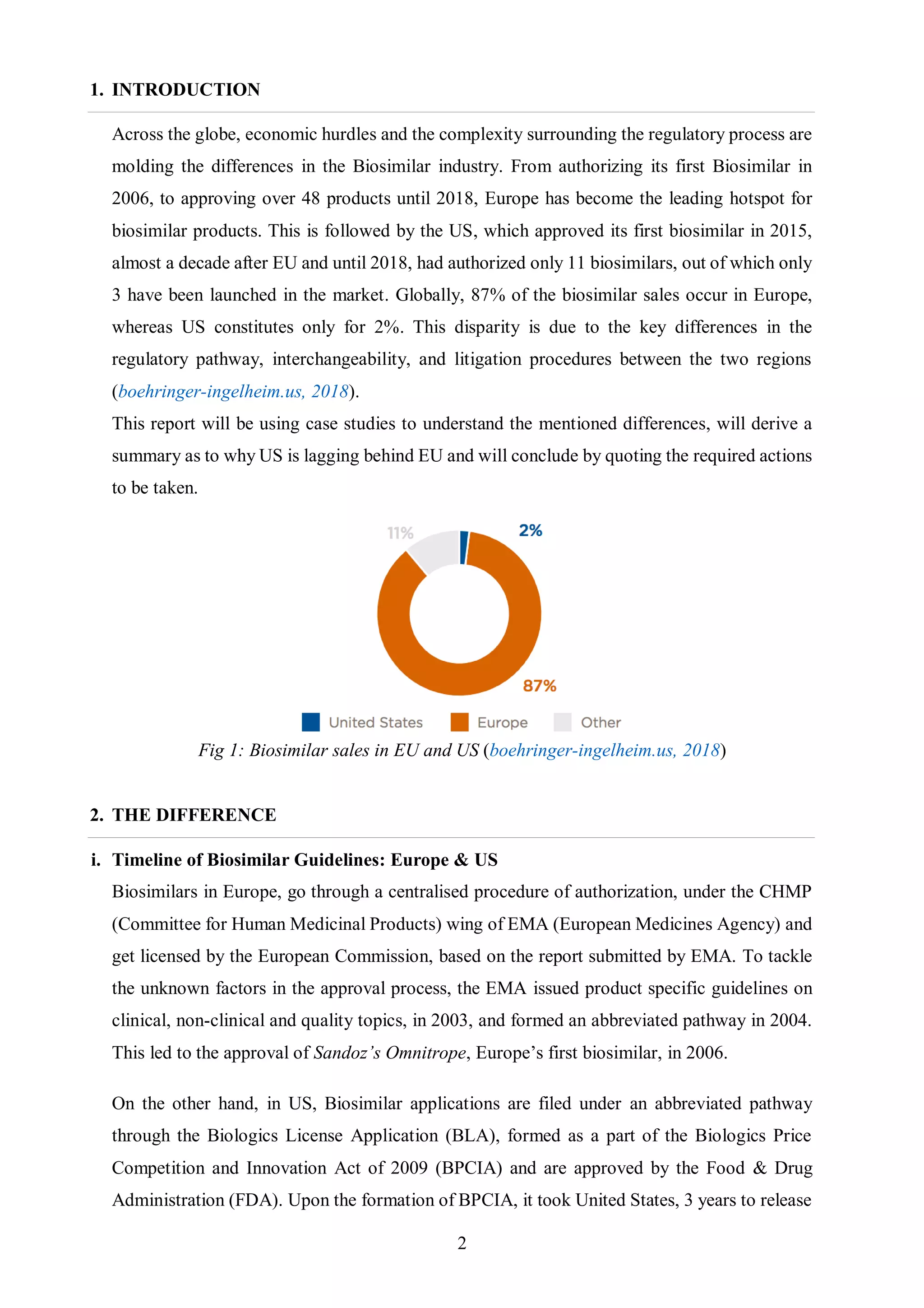
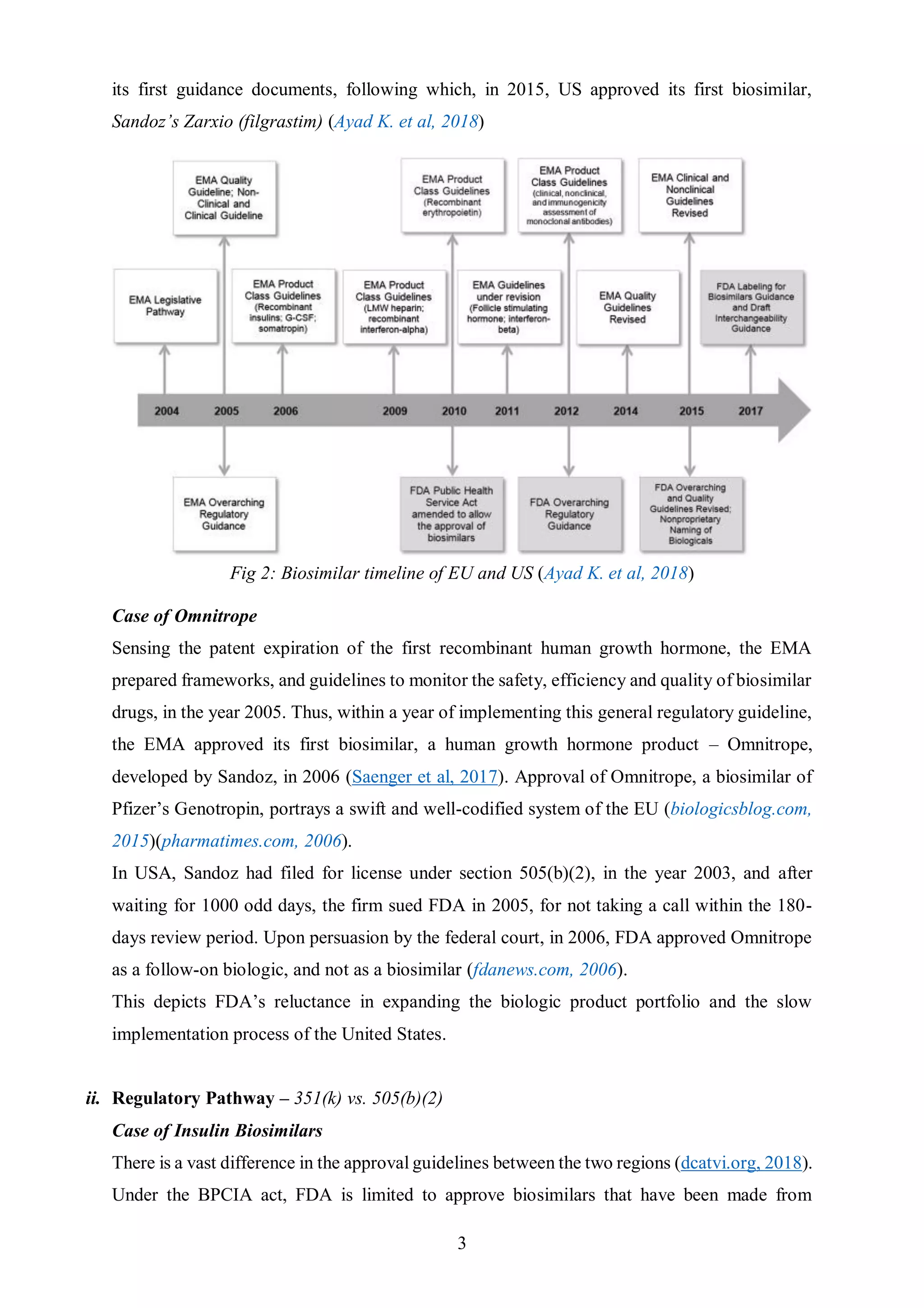
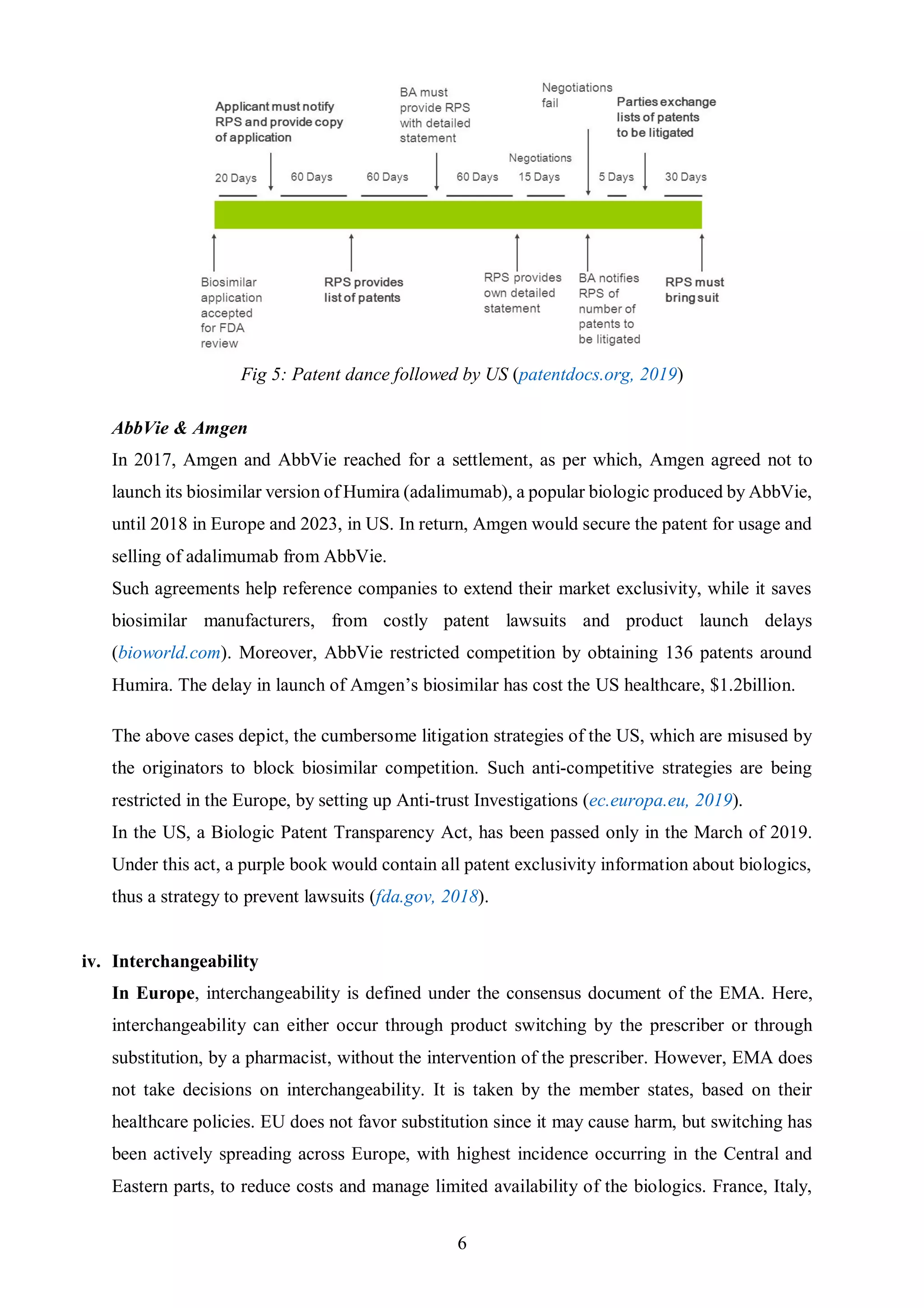
The document discusses key differences between the biosimilar markets in the EU and US. The EU approved its first biosimilar, Omnitrope, in 2006 and has since approved over 48 biosimilars, while the US approved its first, Zarxio, in 2015 and has approved only 11. The disparity is due to differences in regulatory pathways, interchangeability standards, and litigation procedures. The EU has a more established pathway under the EMA and allows for interchangeability through physician switching or pharmacist substitution, while the US pathway was only established in 2009 and has stricter interchangeability requirements. Patent litigation is also more complex and drawn out in the US compared to the centralized process in Europe.





![10
REFERENCES
Ayad K. Ali, Abraham G. Hartzema (2018). Chapter 7 - Post-Authorization Safety Studies for Specialty
Products. Post-Authorization Safety Studies of Medicinal Products, Academic Press, 252-327. doi:
10.1016/B978-0-12-809217-0.00007-6.
Felix, Thomas & B. Jordan, John & Akers, Catherine & Patel, Bina & Drago, Daniela. (2019). Current state of
biologic pharmacovigilance in the European Union: improvements are needed. Expert Opinion on Drug Safety.
18. 10.1080/14740338.2019.1577818.
Jørgensen, Kristin & Olsen, Inge & Goll, Guro & Lorentzen, Merete & Bolstad, Nils & Haavardsholm, Espen &
Lundin, Knut & Mørk, Cato & Jahnsen, Jørgen & K Kvien, Tore. (2017). Articles Switching from originator
infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-
SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. The Lancet. 389. 10.1016/S0140-
6736(17)30068-5.
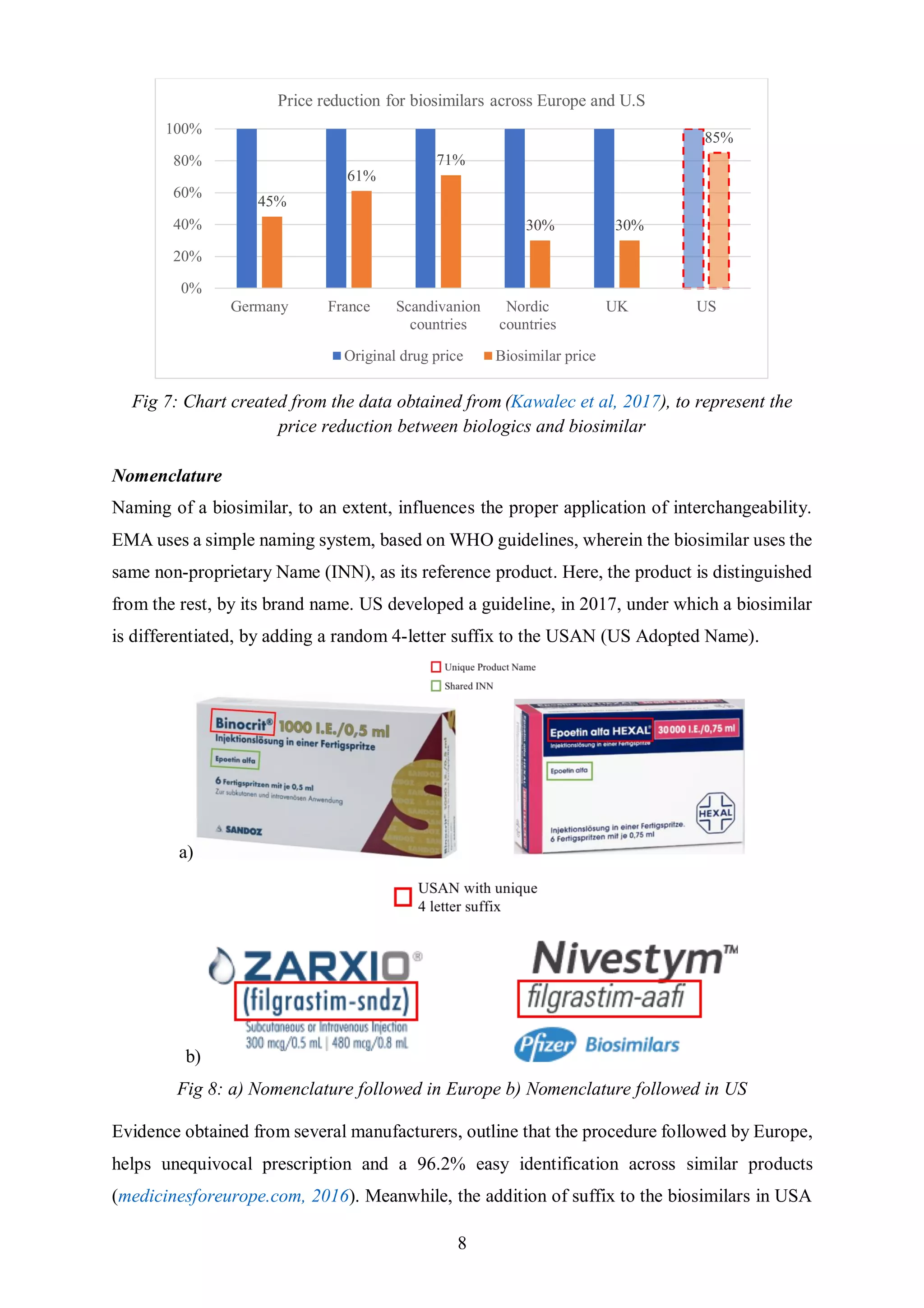
Kawalec, P., Stawowczyk, E., Tesar, T., Skoupá, J., Turcu-Stiolica, A., Dimitrova, M., Petrova, G., Rugaja, Z.,
Männik, A., Harsányi, A., & Draganić, P. (2017). Pricing and Reimbursement of Biosimilars in Central and
Eastern European Countries. Front. Pharmacol..
Malkin, B.J.. (2015). Biosimilars patent litigation in the EU and the US: A comparative strategic overview.
GaBI Journal. 4. 113-117. doi: 10.5639/gabij.2015.0403.026.
MaryII Toufanian, J.D, Capt. Martin Shimer, R.Ph. (2015). Hatch-Waxman 101. Generic Drugs Forum.
Moorkens, E., Vulto, A. G., Huys, I., Dylst, P., Godman, B., Keuerleber, S., … Simoens, S. (2017). Policies for
biosimilar uptake in Europe: An overview. PloS one, 12(12), e0190147. doi:10.1371/journal.pone.0190147
Mulcahy, A. W., Hlavka, J. P., & Case, S. R. (2018). Biosimilar Cost Savings in the United States: Initial
Experience and Future Potential. Rand health quarterly, 7(4), 3.
O’Callaghan, J & P. Barry, S & Bermingham, Margaret & M. Morris, J & Griffin, Brendan. (2018). Regulation
of biosimilar medicines and current perspectives on interchangeability and policy. European Journal of Clinical
Pharmacology. 75. 10.1007/s00228-018-2542-1.
OECD/EU (2018), Health at a Glance: Europe 2018: State of Health in the EU Cycle, OECD Publishing,
Paris/EU, Brussels, https://doi.org/10.1787/health_glance_eur-2018-en.
Rosenstock, J. , Hollander, P. , Bhargava, A. , Ilag, L. L., Pollom, R. K., Zielonka, J. S., Huster, W. J. and
Prince, M. J. (2015), Similar efficacy and safety of LY2963016 insulin glargine and insulin glargine (Lantus®)
in patients with type 2 diabetes who were insulin‐naïve or previously treated with insulin glargine: a
randomized, double‐blind controlled trial (the ELEMENT 2 study). Diabetes Obes Metab, 17: 734-741.
doi:10.1111/dom.12482
Saenger P. (2017). Ten years of biosimilar recombinant human growth hormone in Europe. Drug design,
development and therapy, 11, 1505–1507. doi:10.2147/DDDT.S130317
White, J., & Goldman, J. (2019). Biosimilar and Follow-on Insulin: The Ins, Outs, and Interchangeability.
Journal of Pharmacy Technology, 35(1), 25–35. https://doi.org/10.1177/8755122518802268
www.biologicsblog.com. “Biosimilars Under the 505(b)(2) Pathway (2015). [Online] Available at:
https://www.biologicsblog.com/biosimilars-under-505-b-2-pathway-2. Accessed on: April 30, 2019](https://image.slidesharecdn.com/18200405-topic4-190714154926/75/A-report-outlining-the-differences-in-the-Biosimilar-regulatory-market-between-Europe-and-US-11-2048.jpg)
![11
www.biologicsblog.com. The US Biosimilar Pathway: The First Five Years and What’s Ahead (2015). [Online]
Available at: https://www.biologicsblog.com/u-s-biosimilar-pathway-first-five-years-whats-ahead. Accessed on:
April 28, 2019
www.biopharma-reporter.com. Insulin Biomarkers? US FDA readies market for entry (2018). [Online]
Available at: https://www.biopharma-reporter.com/Article/2018/12/12/Insulin-biosimilars-US-FDA-readies-
market-for-entry. Accessed on: April 30, 2019
www.biosimilarresourcecenter.org. Is Biosimilar Insulin Available? (2015). [Online] Available at:
https://www.biosimilarsresourcecenter.org/faq/biosimilar-insulin-available/. Accessed on: April 28, 2019
www.bioworld.com. Amgen-Abbvie agreement erases uncertainty for Humira biosimilar. [Online] Available at:
http://www.bioworld.com/content/amgen-abbvie-agreement-erases-uncertainty-humira-biosimilar-0. Accessed
on: April 30, 2019
www.blog.jipel.nyu.edu. Sandoz Inc. v. Amgen Inc. and its implications (2018). [Online] Available at:
https://blog.jipel.law.nyu.edu/2018/04/sandoz-inc-v-amgen-inc-and-its-implications/. Accessed on: April 30,
2019
www.boehringer-ingelheim.us. Steps to reducing barriers to biosimilars in the United States (2018). [Online]
Available at: https://www.boehringer-ingelheim.us/sites/us/files/files/barrierstobiosimilars_september2018.pdf.
Accessed on: April 27, 2019
www.dcatvi.org. Biosimilars: Opportunities and Challenges in the US and EU (2018). [Online] Available at:
https://dcatvci.org/5058-biosimilars-opportunities-and-challenges-in-the-us-and-eu. Accessed on: April 28,
2019
www.decisionresourcegroup.com. A Sustainable U.S. Biosimilars Market Requires Sustainable Pricing (2018).
[Online] Available at: https://decisionresourcesgroup.com/drg-blog/sustainable-u-s-biosimilars-market-requires-
sustainable-pricing/. Accessed on: May 01, 2019
www.ec.europa.eu. Competition enforcement in the pharmaceutical sector (2019). [Online] Available at:
http://ec.europa.eu/competition/sectors/pharmaceuticals/report2019/report_en.pdf. Accessed on: April 30, 2019
www.ema.europa.eu. 2017 Annual Report on EudraVigilance for the European Parliament, the Council and the
Commission (2017). [Online] Available at: https://www.ema.europa.eu/en/documents/report/2017-annual-
report-eudravigilance-european-parliament-council-commission-reporting-period-1-january_en.pdf. Accessed
on: May 01, 2019
www.europeanpharmaceuticalreview.com. Biosimilars: Litigation Outlook (2016). [Online] Available at:
https://www.europeanpharmaceuticalreview.com/article/45065/biosimilars-litigation-outlook/. Accessed on:
April 30, 2019
www.fda.gov. FDA Purple Book: Lists of Licensed Biological Products with Reference Product Exclusivity and
Biosimilarity or Interchangeability Evaluations (2018). [Online] Available at:
https://www.fda.gov/drugs/biosimilars/purple-book-lists-licensed-biological-products-reference-product-
exclusivity-and-biosimilarity-or. Accessed on: April 30, 2019
www.fdanews.com. Sandoz granted EC approval for Omnitrope (2006). [Online] Available at:
https://www.fdanews.com/articles/86181-sandoz-granted-ec-approval-for-
omnitrope?utm_source=TrendMD&utm_medium=cpc&utm_campaign=Drug_Daily_Bulletin_TrendMD_0.
Accessed on: April 28, 2019](https://image.slidesharecdn.com/18200405-topic4-190714154926/75/A-report-outlining-the-differences-in-the-Biosimilar-regulatory-market-between-Europe-and-US-12-2048.jpg)
![12
www.forbes.com. What’s holding back Market Uptake of Biosimilars? (2018). [Online] Available at:
https://www.forbes.com/sites/joshuacohen/2018/06/20/whats-holding-back-market-uptake-of-
biosimilars/#31e0c7db691a. Accessed on: April 27, 2019
www.gabionline.net. Establishing interchangeability for biosimilars in US (2018). [Online] Available at:
http://www.gabionline.net/Reports/Establishing-interchangeability-for-biosimilars-in-the-US. Accessed on:
April 30, 2019
www.medicinesforeurope.com. Labelling & Naming (2016). [Online] Available at:
https://www.medicinesforeurope.com/wp-
content/uploads/2016/03/20141203_Grooten_DIA_Berlin_biosimilars.pdf. Accessed on: May 01, 2019
www.patentdocs.org. A solution in search of a problem (2019). [Online] Available at:
https://www.patentdocs.org/followon_biologics/. Accessed on: April 30, 2019
www.pharmaceuticaltechnology.com. FDA’s biosimilars push: will interchangeability requirements change?
(2018). [Online] Available at: https://www.pharmaceutical-technology.com/comment/fdas-biosimilars-push-
will-interchangeability-requirements-change/. Accessed on: April 30, 2019
www.pharmatimes.com. Omnitrope makes history as first ‘biogeneric’ cleared in USA (2006). [Online]
Available at:
http://www.pharmatimes.com/news/omnitrope_makes_history_as_first_biogeneric_cleared_in_usa_996760.
Accessed on: April 28, 2019
www.raps.org. Pharma companies warn of Regulatory ‘Dead Zone’ with FDA’s Interpretation of BPCIA
(2016). [Online] Available at: https://www.raps.org/regulatory-focus%E2%84%A2/news-
articles/2016/5/pharma-companies-warn-of-regulatory-%E2%80%98dead-zone%E2%80%99-with-
fda%E2%80%99s-interpretation-of-bpcia. Accessed on: April 30, 2019
www.specialtypharmacytimes.com. The high cost of delays in biosimilars hitting the US market (2015).
[Online] Available at: https://www.specialtypharmacytimes.com/news/the-high-cost-of-delays-in-biosimilars-
hitting-the-us-market. Accessed on: April 30, 2019](https://image.slidesharecdn.com/18200405-topic4-190714154926/75/A-report-outlining-the-differences-in-the-Biosimilar-regulatory-market-between-Europe-and-US-13-2048.jpg)