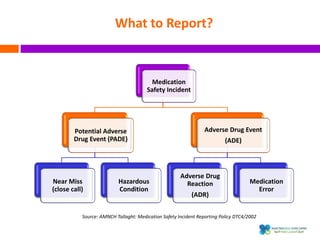
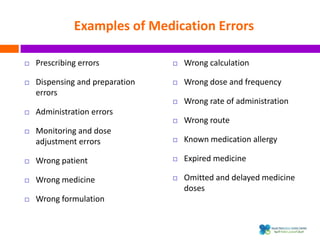
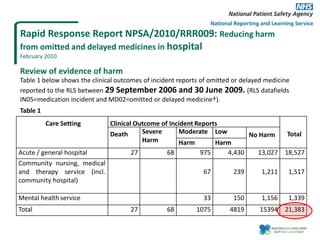
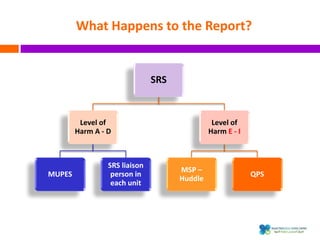
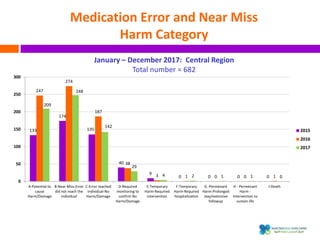
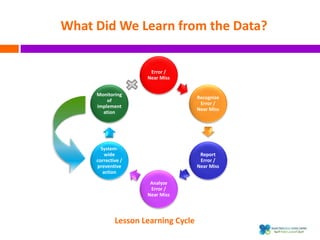
The document outlines a certification course on medication error and near miss reporting at King Saud Bin Abdulaziz University aimed at enhancing medication safety. It highlights the importance of reporting incidents, the types of reportable events, and the ethical obligations involved, along with statistical outcomes from reported incidents. It also emphasizes a culture of learning from errors and provides a framework for categorizing and addressing medication-related incidents.