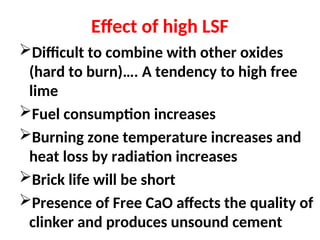
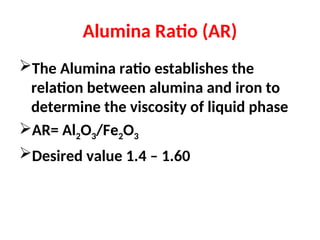
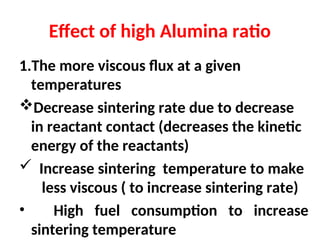
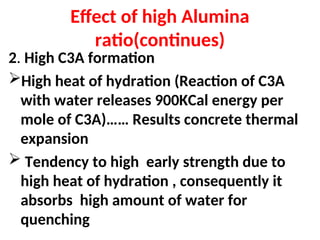
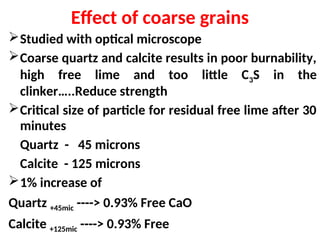
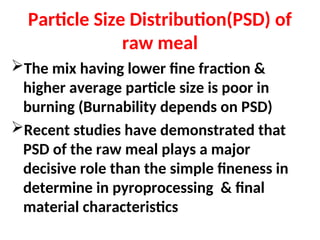
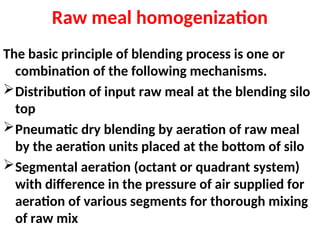
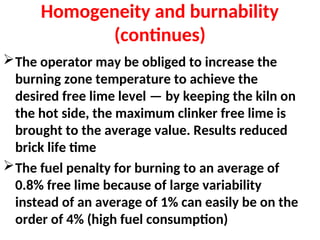
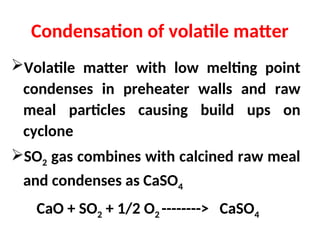
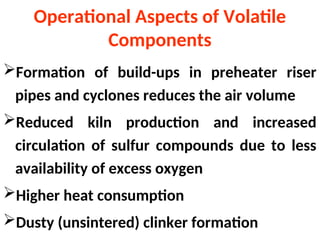
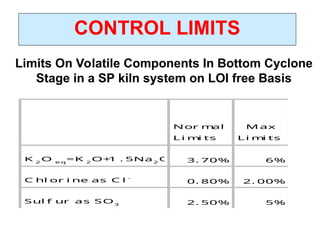
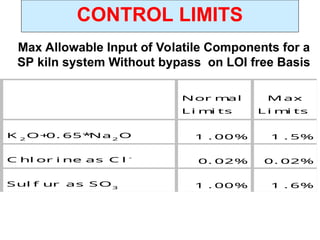
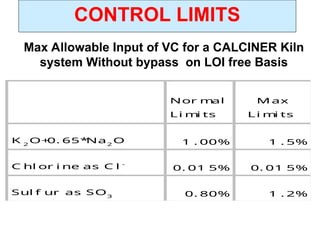
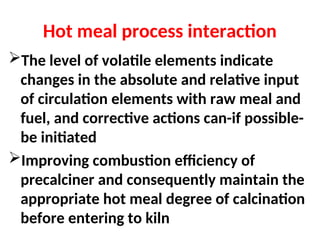
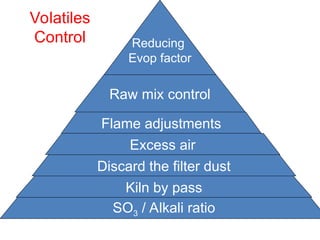
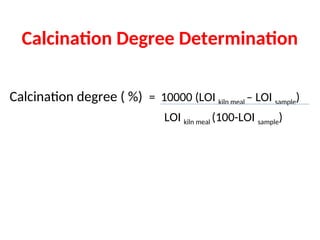
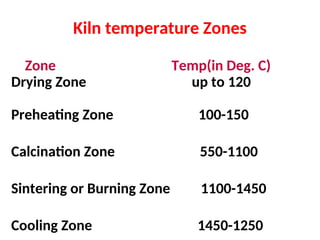
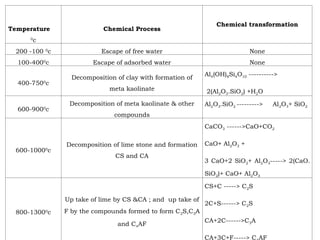
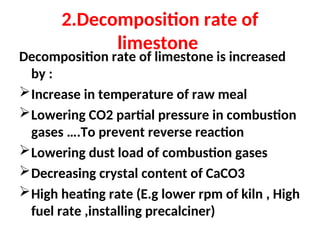
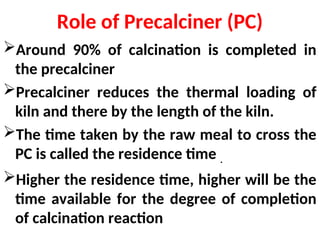
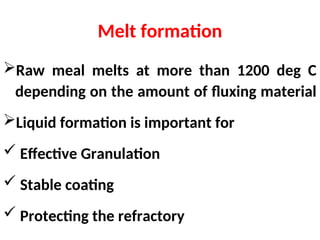
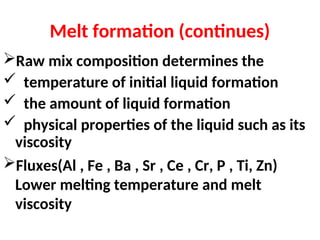
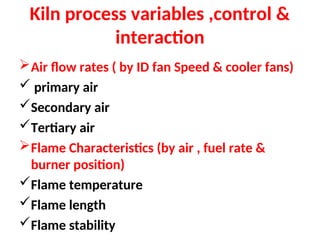
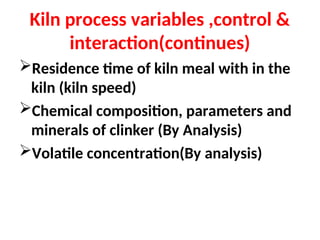
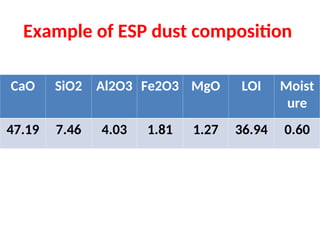
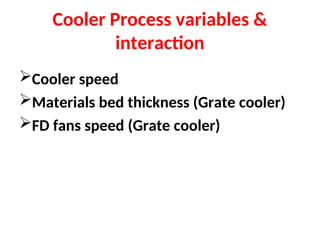
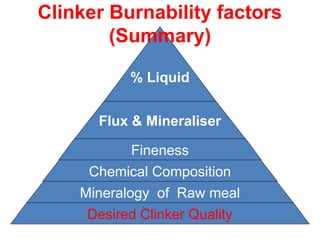
The document outlines the quality control measures and objectives related to raw meal and clinker in cement manufacturing. It discusses various factors affecting cement quality, including chemical composition, raw mix design, and the importance of raw meal fineness and homogeneity. Additionally, it delves into the manufacturing process, testing techniques, and the relationship between raw meal properties and clinker production efficiency.













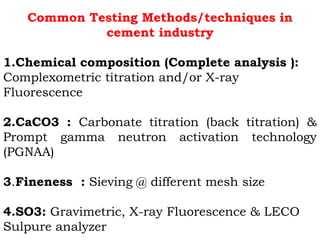
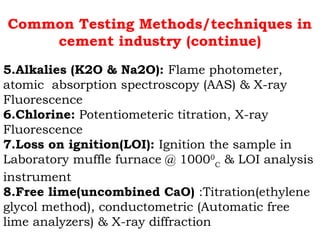














![Lime saturation factor (LSF)
The amount of CaO which is enough to
saturate or combine SiO2, Al2O3, and Fe2O3 to
form Portland cement clinker
LSF= [CaO / (2.8SiO2+1.2Al2O3+0.65Fe2O3)]*100
Desired value 92-98 %](https://image.slidesharecdn.com/567128122-raw-meal-clinker-quality-control-240903184018-9bed5c2a/85/567128122-Raw-Meal-Clinker-Quality-Control-ppt-39-320.jpg)