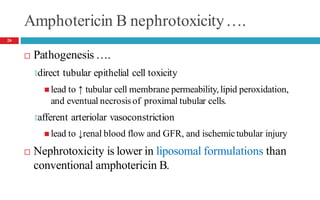
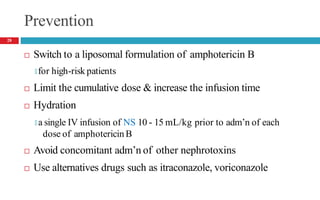
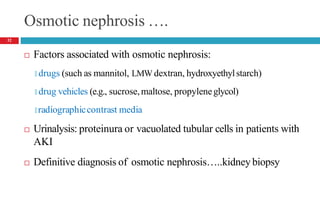
Drug-induced kidney disease (DIKD) can have various presentations depending on the drug and clinical setting. Studies show that 20-30% of hospital-acquired acute kidney injury (AKI) cases are associated with nephrotoxic medications such as aminoglycosides, iodinated contrast media, and NSAIDs. DIKD most commonly manifests as acute tubular necrosis, characterized by rises in serum creatinine and BUN, along with urinary abnormalities. Prevention focuses on avoiding unnecessary nephrotoxic drugs, proper dosing based on kidney function, and adequate hydration. Management involves discontinuing causative agents and providing supportive care.
















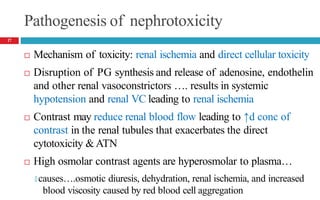
![Risk factors
Preexisting kidney disease [GFR <60mL/min/1.73 m2]
Conditions associated with decreased renal blood flow
🞑 CHF, dehydration/volume depletion, hypotension
Patients with atherosclerosis
Diabetes, due to coexisting kidney disease (diabetic nephropathy)
Larger volumes or doses of contrast
Use of high osmolar contrast agents
Concurrent use of nephrotoxins, NSAIDs and ACEIs
Risk factors are additive
18](https://image.slidesharecdn.com/4-dikd-20222-221226083155-a46a50e0/85/4-DIKD-2022-2-pptx-18-320.jpg)