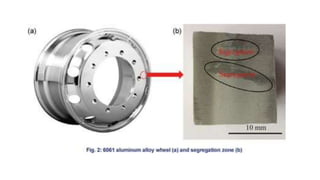
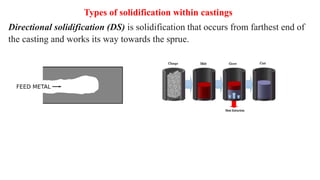
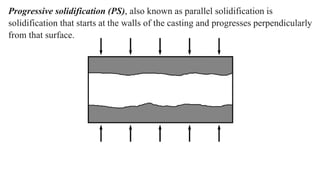
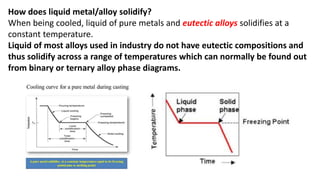
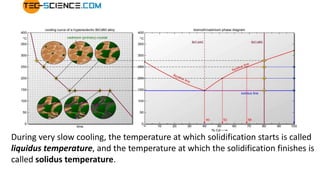
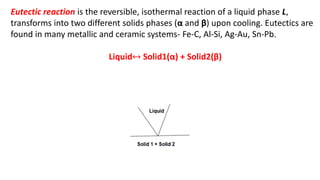
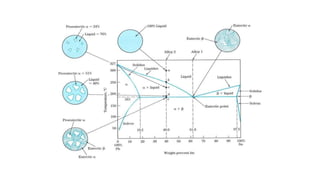
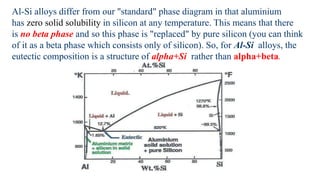
The document covers advanced topics in solidification processing of light metals and alloys, including dendrite formation, microsegregation, and the distinction between eutectic and non-eutectic alloys. It discusses techniques like high pressure gas atomization and spray forming for producing fine metal powders and near-net shape components, emphasizing their significance in enhancing mechanical properties and achieving complex material characteristics. Additionally, the challenges and methods of preventing segregation during solidification are explored, highlighting the importance of solidification in determining material properties.