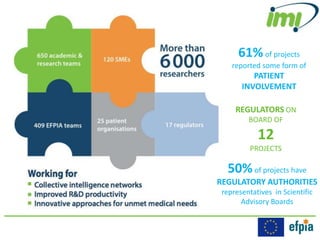
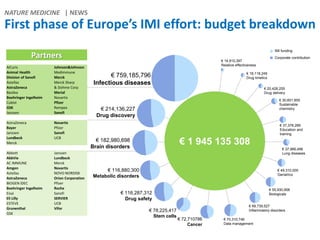
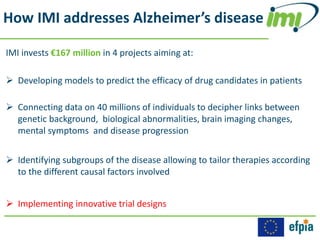
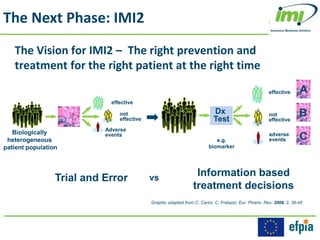
The Innovative Medicines Initiative (IMI) promotes open collaboration in public-private consortia focused on various health challenges, including diabetes and cancer, with approximately 50% of its projects involving regulatory authorities and 61% including patient input. IMI's strategic agenda, labeled IMI2, aims to enhance drug efficacy prediction and tailor therapies for Alzheimer's and other neurodegenerative diseases, supported by a budget of €3.45 billion jointly funded by the EU and the pharmaceutical industry. Key partnerships and funding allocations reflect a broad commitment to advancing drug discovery and patient outcomes through collaborative research.