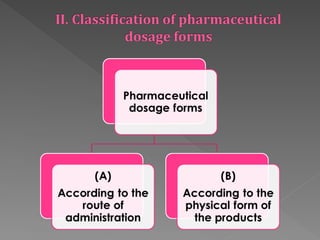
The document provides an extensive overview of pharmaceutics, covering definitions, functions, and classifications of dosage forms and pharmaceutical additives. It details the various types of dosage forms, including liquid, solid, and semi-solid forms, and discusses their importance for drug stability, administration, and patient adherence. Additionally, it highlights methods of preparation and issues related to stability and preservation of pharmaceutical solutions.