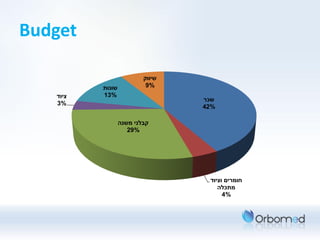
Orbomed is developing an office-based hysteroscopy product line to enable early diagnosis and timely treatment of intrauterine pathology. Their team includes medical and business experts. Hysteroscopy is currently the gold standard for detecting intrauterine issues but is often painful without anesthesia. Orbomed aims to improve patient comfort, eliminate general anesthesia need, shorten procedures, and reduce costs. Their product will include 3D scissors and a tissue sampling tool. Within 24 months, they plan to develop a working prototype and budget $750k for research and development. Their target customers are gynecology clinics and potential applications include polyp removal, scar tissue treatment, and biopsies. The global market for related medical devices is over