This study investigated the effects of soft diet and omega-3 fortified soft diet on neurogenesis in the dentate gyrus and subventricular zone of rats. Thirty rats were divided into three groups: a control group fed a hard diet, a group fed a soft diet, and a group fed a soft diet fortified with omega-3 fatty acids. Immunohistochemistry was used to detect bromodeoxyuridine-labeled proliferating cells at various time points. Results showed that the hard diet and omega-3 fortified soft diet increased neurogenesis in the dentate gyrus compared to the soft diet. In the subventricular zone, the omega-3 fortified soft diet increased proliferation and survival
![Immunohistochemical study on the effect of soft diet and
omega 3-fortified soft diet on neurogenesis in the rat dentate
gyrus and the subventricular zone
Rehab Ahmed Rifaai, Nashwa Fathy El-Tahawy and Entesar Ali Saber
Department of Histology, Faculty of Medicine, Minia
University, Egypt
Correspondence to Rehab Ahmed Rifaai, Department
of Histology, Faculty of Medicine, Minia University,
Egypt
Tel: + 103358376; fax: + 86 2342813;
e-mail: rehabrifaai@yahoo.com
Received 24 January 2011
Accepted 23 April 2011
The Egyptian Journal of Histology
2011, 00:000–000
Background
Adult brain neurogenesis persists in the subventricular zone (SVZ) and in the
subgranular zone (SGZ) of the dentate gyrus. Modulation of neurogenesis by diet is a
mechanism by which nutrition affects memory, learning, and mood.
Aim of the study
To study the effect of the soft diet with or without omega 3 fatty acids on neurogenesis.
Materials and methods
Thirty weaned male albino rats (3 weeks) were divided into three groups. Group 1
(control group) were fed on hard diets, group 2 were fed on soft diets, and group 3
were fed on soft diets plus omega 3 fatty acids for 3 months. Nerve cell proliferation in
the SVZ and the SGZ was detected immunohistochemically using thymidine analog
bromodeoxyuridine (BrdU). The results were statistically analyzed.
Results
In the dentate gyrus, there was a significant increase in the number of BrdU-positive
cells in groups 1 and 3 compared with group 2. Meanwhile in the SVZ, there was a
significant increase in the number of BrdU-positive cells in group 3 compared with
group 1. In group 1, the newly formed cells in the SGZ reached the granular cell layer
of the dentate gyrus. The newly formed cells in the SVZ reached the olfactory bulb
(OB) after 2 weeks but failed to survive for 4 weeks in the OB. In group 2, few newly
formed cells reached the granular cell layer of the dentate gyrus, but they failed to
reach the OB. In group 3, the newly formed cells reached their destination in the
granular cell layer of the dentate gyrus and the OB. In the OB, the cells succeeded to
survive for 4 weeks and were incorporated among the granular cells of OB.
Conclusion
Hard diet and omega 3-fortified soft diet had a stimulatory effect on the process of
neurogenesis in the dentate gyrus. Meanwhile in the SVZ, fortified soft diet had more
stimulatory effect on proliferation and improvement of the survival rate of the newly
formed cells than the hard diet.
Keywords:
dentate gyrus, diet, immunocytochemical, neurogenesis, subventricular zone
Egypt J Histol 00:000–000
c 2011 The Egyptian Journal of Histology
1110-0559
Introduction
Adult stem cells are present in many tissues, including
bone marrow, skin, gastrointestinal tract, muscle, adipose
tissue, and brain [1]. Neurogenesis is a life-long
occurrence that is limited to specific sites within the
brain, namely, the subventricular zone (SVZ) and the
subgranular zone (SGZ) of the hippocampus [2].
Neurogenesis is a complex multistage and multiweek
process involving proliferation, neuronal differentiation
and, ultimately, survival and integration into circuitry [3].
The integration of adult-born neurons into the circuitry
of the adult hippocampus suggests an important role for
adult hippocampal neurogenesis (AHN) in learning and
memory [4]. Nutrition affects brain function [5].
Modulation of AHN by diet emerges as a possible
mechanism by which nutrition impacts mental health.
Reduction of masticatory afferent stimuli due to long-
term soft diet fed may induce neuronal loss in the
hippocampus and reduce memory/learning ability [6].
The omega 3 fatty acids are found in the diet as doco-
sahexaenoic acid (22 : 6n-3, DHA), a-linolenic acid (18 : 3
omega-3), and eicosapentaenoic acid (20 : 5 omega-3).
Salmon, flax seeds, and walnuts are excellent sources of
omega 3 fatty acids. Very good sources of these healthy
fats include cauliflower, cabbage, cloves, and mustard
seeds. Good sources of these fats include halibut, shrimp,
cod, tuna, soybeans, tofu, kale, collard greens, and
brussels sprouts [7]. Each of the omega 3 fatty acids
has different functions in different cells. DHA is found
predominantly in neuronal membranes in the gray matter
and constitutes a major component of the brain [8]. It
plays important roles functionally and structurally [9].
Original article 1
1110-0559 c 2011 The Egyptian Journal of Histology DOI: 10.1097/01.EHX.0000399683.51243.c2](https://image.slidesharecdn.com/2-neurogenesis-150318163022-conversion-gate01/85/2-neurogenesis-1-320.jpg)
![Depletion of DHA from brain and retina interferes with
normal neurogenesis and neurological function [10].
Bromodeoxyuridine (BrdU) is a thymidine analog that
incorporates DNA of dividing cells during the S-phase of
the cell cycle. As such, BrdU is used for birth dating and
for monitoring cell proliferation. BrdU can be detected by
immunohistochemistry, using a monoclonal antibody
directed against single-stranded DNA containing BrdU
[11]. BrdU immunohistochemistry has been instrumental
for the study of the development of the nervous system
and to confirm that neurogenesis occurs in the adult
mammalian brain, including human [12]. It was
interesting to study the effect of fortification of the soft
diet with omega 3 fatty acid on neurogenesis in
comparison with the hard diet.
Materials and methods
Animals
This study was carried out according to the protocols
approved by the Animal Care and Use Committee at the
Minia University Animal House (Egypt). This study was
carried out on 30 male albino pups, which were weaned at
3 weeks after birth. The pups were divided into three
groups of 10 pups each.
Group 1: the control group (hard diet-fed group)
The animals were fed on pelleted chow for 3 months.
Group 2 (soft diet-fed group)
The animals were fed on powdered and wetted chow with
the same ingredients as that of the previous group for 3
months.
Group 3 (omega 3-fortified soft diet-fed group)
The animals were fed on powdered chow containing the
same ingredients but with the addition of omega 3 fatty
acid (35 mg/kg) for 3 months [13].
Bromodeoxyuridine administration
At the end of experiment, rats of groups 1, 2, and 3
received intraperitoneal injection with BrdU (50 mg/kg)
dissolved in PBS every 12 h for 3 consecutive days [14].
The animal were killed 2 days, 2 weeks, and 4 weeks after
the last BrdU injection. The number of BrdU-positive
cells after 2 days indicates the proliferation rate. The
number of BrdU-positive cells after 2 weeks shows the
migration of the newly formed cells, whereas the number
of BrdU-positive cells after 4 weeks indicates the survival
rate [15].
Immunohistochemistry
Brains were removed and fixed in 4% paraformaldehyde
and then processed for paraffin sectioning. Sections were
cut at 10 mm. The sections were immunostained with a
monoclonal BrdU antibody (1 : 500; Biodesign Inc., Sigma
Aldrich, Egypt). Immunohistochemical staining was
performed according to a previously published protocol
[16] as follows:
(1) Sections were deparaffinized, hydrated and then
washed in phosphate buffered saline (PBS)
(0.1 mol/l);
(2) Sections were pretreated with hydrogen peroxide (1%
H2O2 for 2 min) to eliminate endogenous peroxidase;
(3) Sections were incubated in 4 N HCl for 30 min at
room temperature (for DNA denaturation);
(4) Sections were immersed in trypsin and PBS (1 mg/
ml) for 10 min at 371C. After the acid washes, borate
buffer (0.1 mol/l) was added to buffer the cells for
12 min at room temperature;
(5) Sections were incubated with 1% Triton X-100 (0.1 M
PBS, pH = 7.4) solution containing rat anti-BrdU
antibody (1 : 500). Sections were incubated overnight
at room temperature. After incubation with the
primary antibodies, the sections were rinsed with
PBS and subsequently incubated in a biotinylated
goat anti-rat IgG secondary antibody (Vector Labora-
tory, 1 : 2000) for 1 h at room temperature;
(6) Sections were then incubated for 30min in the Vecta-
stain ABC reagent. Diaminobenzidine was used as a
chromagen [17].
Bromodeoxyuridine-positive cells counts
The morphometric measurements were taken using
a Leica Quin 500C image analyzer computer system
(Leica Imaging system Ltd., Cambridge, England). In
the dentate gyrus, cells were counted in the field of a
40 Â objective using light microscope; BrdU-positive
cells were counted throughout the entire SGZ and the
granule cell layer of the dentate gyrus. In each animal, six
sections were counted and the distance between sections
was 300 mm to scan through the depth of the hippocampus.
In the SVZ, cells were counted in the field of the oil
immersion lens. Cells were counted in 10 adjacent
nonoverlapping fields. In each animal, six sections were
counted and the distance between sections was 300 mm.
Statistical analysis
Statistical analysis was carried out using the Statistics
Package instat. Statistical significance of the experiments
was determined using the one-way analysis of variance test
followed by the Tukey–Cramer posthoc test. A P value less
than 0.05 was considered as statistically significant.
Results
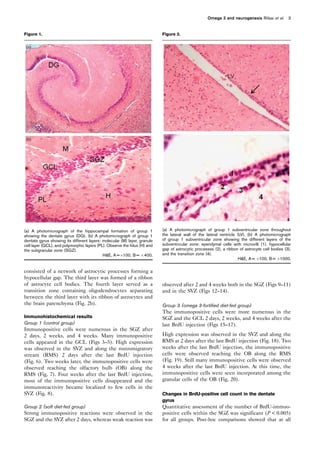
The dentate gyrus is one component of the hippocampal
formation (Fig. 1a). The dentate gyrus consists of the
molecular layer, the GCL, and the polymorphic layers.
The concavity of the dentate gyrus is termed the hilus.
Some ectopic granule cells are located within the hilus.
The SGZ is the area of two cell bodies’ width between
the GCL and the hilus (Fig. 1b).
The SVZ is a paired brain structure situated throughout
the lateral walls of the lateral ventricles (Fig. 2a). It has
four distinct layers. The innermost layer consisted of a
single layer (monolayer) of ependymal cells with
microvilli lining the ventricular cavity. The second layer
2 The Egyptian Journal of Histology](https://image.slidesharecdn.com/2-neurogenesis-150318163022-conversion-gate01/85/2-neurogenesis-2-320.jpg)
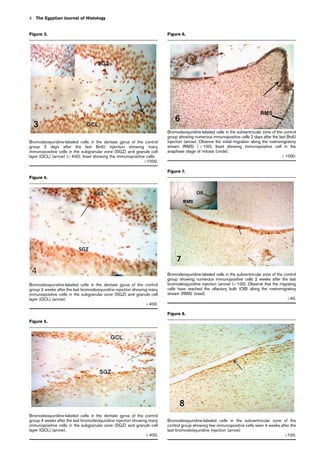
![Changes in BrdU-positive cell count in the SVZ
The BrdU-labeled cells in the SVZ of the hard and the
omega 3-fortified soft diet-fed groups were significantly
higher (P 0.05) than the soft diet-fed group 2 days, 2
weeks, and 4 weeks after the last BrdU injection. On
comparing the number of the immunopositive cells in the
hard diet and the omega 3-fortified soft diet-fed groups, it
was found that the number of the immunopositive cells was
significantly higher (P0.05) in the omega 3-fortified soft
diet-fed group than in the hard diet-fed group 2 days, 2
weeks, and 4 weeks after the last BrdU injection (Graph 1b).
Discussion
This study demonstrated that neurogenesis persists in the
SVZ and in the SGZ of adult rat brain. More BrdU-
immunopositive cells were observed in both areas 2 days
after the last BrdU injection than after 2 weeks and 4 weeks
in all the examined groups. The reduction in the number of
the immunopositive cells along the time course might be as
a result of the death of some newly born cells. Hard diet and
omega 3-fortified soft diet increased neuronal proliferation
and survival in both areas in comparison with the soft
diet alone. Both the proliferation and the survival rate of the
newly born cells were better in the omega 3-fortified soft
diet-fed group. In the omega 3-fortified soft diet-fed group,
the newly born neurons succeeded in reaching their
destination either in the GCL or in the OB.
Neuronal precursors cells are self-renewing, with the
potential to differentiate into all three basic cell types in
the central nervous system (CNS), including neurons, oligo-
dendrocytes, and astrocytes [18]. Neuronal progenitors in
the SVZ migrate to the OB along the RMS. After the
newborn neurons reach the middle of the OB, they detach
from the chain and migrate radially. They differentiate into
granule and periglomerular neurons [19]. These modulate
the tuning of bulbar activity to enhance olfactory
discrimination performance and potentially regulate the
categorization of novel odorants [20].
Many precursors in the SGZ die within 2 weeks [21].
Living precursors in the SGZ migrate into the dentate GCL
where the majority ultimately acquire morphological
characteristics of granule cells and express neuron-specific
markers [22]. In the GCL, they develop synapses and axonal
projections to receive and deliver signals, respectively, and
hence they can execute their function [23].
Food texture has an impact on AHN, rats fed with a soft
diet, as opposed to a solid diet, exhibit decreased
hippocampal progenitor cell proliferation [24]. The
decrease of neurogenesis in the soft diet-fed rats had
been explained by the emotional stress observed in those
rats that led to increased level of corticosterone [25].
Corticosterone is a common downregulator of neurogenesis
[26]. Corticosterone reduces the level of brain-derived
neurotrophic factor (BDNF) mRNA through the
glucocorticoid receptor located in the dentate gyrus [27].
BDNF is a member of a family of related neurotrophic
proteins. It is a positive regulator of both proliferation and
survival of neurons. It also prevents neurons from dying
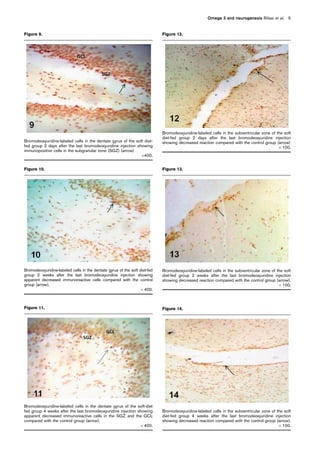
Figure 20.
Bromodeoxyuridine-labeled cells in the subventricular zone of the
omega 3-fortified soft diet-fed group showing that many
immunopositive cells are present 4 weeks after the last
bromodeoxyuridine injection (arrow) (Â 400). Observe some
immunopositive cells grouped into nests (upper inset). Lower inset
showing the immunopositive cells incorporated among cells in the
granular cell layer of the olfactory bulb (OB)
Â1000.
Graph 1.
Mean + standard deviation (SD) bar graphs of bromodeoxyuridine
(BrdU) immunopositive cell counts of (a) subgranular zone and (b)
subventricular zone showing significant increase in the number of
bromodeoxyuridine-positive cells in the omega 3-fortified soft diet-fed
group when compared with both soft diet and hard diet-fed groups.
Omega 3 and neurogenesis Rifaai et al. 7](https://image.slidesharecdn.com/2-neurogenesis-150318163022-conversion-gate01/85/2-neurogenesis-7-320.jpg)
![during development [28]. BDNF level increased in the
brain after voluntary physical activity, including masticatory
activity. This might explain why hard diet enhances
neurogenesis. BDNF infusion in the lateral ventricles was
found to augment SVZ neurogenesis [29]. In contrast,
administration of BDNF into the lateral ventricles led to a
decrease in SVZ neurogenesis in rat [30].
DHA is an omega 3 fatty acid highly enriched in the CNS
and is critical for brain development and function. DHA
improves both neuronal proliferation and survival. This
observation is consistent with the findings of recent studies
in rats fed with DHA [31]. DHA significantly enhances
hippocampal neurogenesis in the transgenic fat-1 mice rich
in endogenous DHA. DHA can influence cell function
through multiple mechanisms. DHA esterified into
phospholipids of the plasma membrane bilayer signi-
ficantly alters many basic membrane properties, including
fluidity, flexibility, permeability, electrostatic behavior, and
consequently regulates the neurotransmission and signal
transduction [32]. However, the unesterified free-DHA
exerts complex changes in gene expression in the brain,
including the expression of genes involved in neurogenesis
[33]. Omega 3 regulates corticotrophin factor, increases
seretonergic function, increases dentritic arborization,
prevents neural apoptosis, improves cerebral blood flow,
and regulates gene expression [34]. Omega 3 fatty acids are
the most efficient for the development of adequate brain
cell membranes and intercellular neuronal connections [35].
AHN affects learning and memory [36]. Newborn neurons
that are young when events occur have a specialized role in
encoding, in storage, and in temporally relating one event to
another, explaining a possible requirement of newborn
neurons in the process of learning and memory [37].
Neurodegenerative diseases such as Alzheimer’s disease and
Parkinson’s disease affect AHN either by stimulation or by
inhibition. AHN is also influenced by pathological
conditions. For example, it is increased in epilepsy and
stroke and decreased in HIV infection. CNS inflammation
affects the integration of newborn neurons into circuits [38].
Conclusion
In conclusion, the following observations are presented:
(1) Hard diet has a stimulatory effect on the process of
neurogenesis through the masticatory activity;
(2) In contrast, soft diet alone has an inhibitory effect on
the process of neurogenesis;
(3) If there is no escape from using soft diet, it might be
fortified with omega 3 fatty acid to obtain a better
effect on neurogenesis.
(4) The omega 3-fortified soft diet is suggested to
replace the insufficient masticatory activity.
References
1 Bachstetter AD, Jernberg J, Schlunk A, Vila JL, Hudson C, Cole MJ, et al.
Spirulina promotes stem cell genesis and protects against LPS
induced declines in neural stem cell proliferation. PloS One 2010;
5:e10496.
2 Clelland CD, Choi M, Romberg C, Clemenson GD Jr, Fragniere A, Tyers P,
et al. A functional role for adult hippocampal neurogenesis in spatial pattern
separation. Science 2009; 325:210–213.
3 Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of
adult-generated granule cells into spatial memory networks in the dentate
gyrus. Nat Neurosci 2007; 10:355–362.
4 Deng W, Aimone JB, Gage FH. New neurons and new memories: how does
adult hippocampal neurogenesis affect learning and memory? Nat Rev
Neurosci 2010; 11:339–350.
5 Van Praag H. Exercise and the brain: something to chew on. Trends Neurosci
2009; 32:283–290.
6 Tsutsui K, Kaku M, Motokawa M, Tohma Y, Kawata T, Fujita T, et al. Influences
of reduced masticatory sensory input from soft-diet feeding upon spatial
memory/learning ability in mice. Biomed Res 2007; 28:1–7.
7 Chung H, Nettleton JA, Lemaitre RN, Barr RG, Tsai MY, Tracy RP, Siscovick
DS. Frequency and type of seafood consumed influence plasma (n-3) fatty
acid concentrations. J Nutr 2008; 138:2422–2427.
8 Yehuda S, Rabinovitz S, Mostofsky DI. Essential fatty acids and the brain:
from infancy to aging. Neurobiol Aging 2005; 26 (Suppl 1):98–102.
9 Ohkubo T, Tanaka Y. Administration of DHA-PS to aged mice was suitable
for increasing Hippocampal PS and DHA ratio. J Oleo Sci 2010; 59:
247–253.
10 Innis SM. Omega-3 fatty acids and neural development to 2 years of age: do
we know enough for dietary recommendations? J Pediat Gastroenterol Nutr
2009; 48 (Suppl 1):S16–S24.
11 Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: a
new reagent for detection of DNA replication. Science 1982; 218:474–475.
12 Rakic P. Adult neurogenesis in mammals: an identity crisis. J Neurosci; 2002;
22:614–618.
13 Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic
acid-mediated neuroprotection in a rat model of transient, focal cerebral
ischemia. Stroke 2009; 40:3121–3126.
14 So JK, Tae GS, Hee RP, Park M, Kim MS, Hyung SK, et al. Curcumin
stimulates proliferation of embryonic neural progenitor cells and
neurogenesis in the adult hippocampus. J Biol Chem 2008; 283:
14497–14505.
15 Taupin P. BrdU immunohistochemistry for studying adult neurogenesis:
paradigms, pitfalls, limitations and validation. Brain Res Brain Res Rev 2007;
53:198–214.
16 Coˆte´ S, Ribeiro da Silva A, Cuello AC. Current protocols for light microscopy
immunocytochemistry. In: Cuello AC, editor. Immunohistochemistry.
Chichester: John Wiley Sons Ltd; 1993. pp. 147–168.
17 Risio M, Candelaresi G, Rossini FP. Bromodeoxyuridine uptake and
proliferating cell nuclear antigen expression throughout the colorectal
tumor sequence. Cancer Epidemiol Biomarkers Prev 1993; 2:363–367.
18 Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus
develop essential properties of functional CNS neurons. Nat Neurosci 2002;
5:438–445.
19 Lois C, Alvarez Buylla A. Long-distance neuronal migration in the adult
mammalian brain. Science 1994; 264:1145–1148.
20 Cecchi GA, Petreanu LT, Alvarez Buylla A, Magnasco MO. Unsupervised
learning and adaptation in a model of adult neurogenesis. J Comput Neurosci
2001; 11:175–182.
21 Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early
determination and long-term persistence of adult-generated new neurons in
the hippocampus of mice. Development 2003; 130:391–399.
22 Farmer J, Zhao X, Van Praag H, Wodtke K, Gage FH, Christie BR. Effects of
voluntary exercise on synaptic plasticity and gene expression in the dentate
gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 2004;
124:71–79.
23 Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send
axonal projections to field CA3 and are surrounded by synaptic vesicles.
J Comp Neurol 1999; 406:449–460.
24 Aoki H, Kimoto K, Hori N, Toyoda M. Cell proliferation in the dentate gyrus of
rat hippocampus is inhibited by soft diet feeding. Gerontology 2005;
51:369–374.
25 Yamamoto T, Hirayama A, Hosoe N, Furube M, Hirano S. Soft-diet feeding
inhibits adult neurogenesis in hippocampus of mice. Bull Tokyo Dent Coll
2009; 50:117–124.
26 Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids
in the dentate gyrus. Neuroscience 1994; 61:203–209.
27 Schaaf MJ, De Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF
expression in the hippocampus. Implications for memory formation. Stress
2000; 3:201–208.
28 Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to
network and physiology. Physiol Rev 2005; 85:523–569.
29 Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-
derived neurotrophic factor induces both neostriatal and olfactory neuronal
recruitment from endogenous progenitor cells in the adult forebrain.
J Neurosci 2001; 21:6718–6731.
8 The Egyptian Journal of Histology](https://image.slidesharecdn.com/2-neurogenesis-150318163022-conversion-gate01/85/2-neurogenesis-8-320.jpg)

