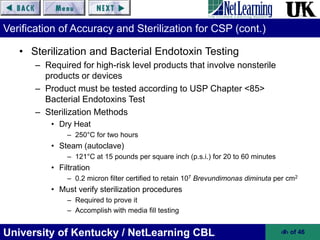
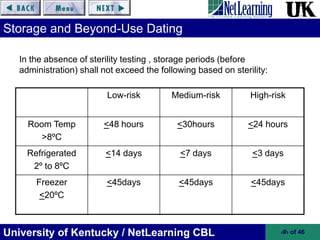
This document provides an overview of key sections of USP Chapter <797> on pharmaceutical compounding of sterile products. It discusses requirements for personnel training and certification, environmental quality controls, and risk classifications for compounded sterile preparations. Proper cleaning and garbing of personnel, accuracy verification of compounded products, and sterilization methods are also summarized. The goal of Chapter <797> is to prevent patient harm from microbial contamination or incorrect ingredients in compounded sterile products.