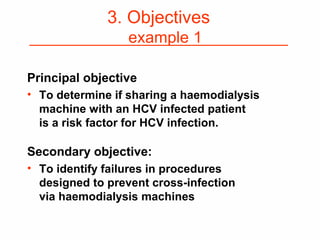
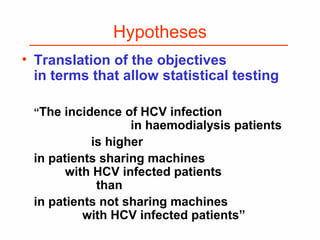
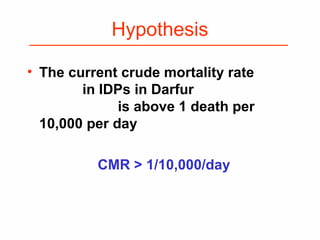
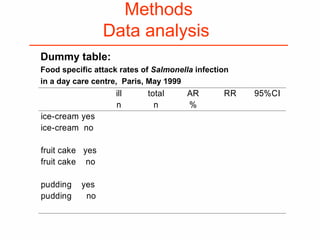
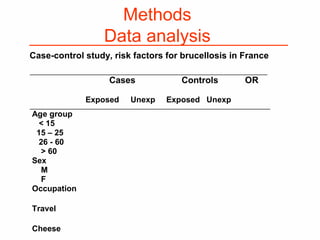
This document provides guidance on how to write an effective study protocol. It explains that a study protocol should describe each step of the study to check that the objectives can be achieved and the study is feasible. It also ensures all relevant information is collected and rules are established for partners. The document recommends following a checklist of key items and obtaining examples from similar studies. It then outlines the typical sections of a study protocol, including background, objectives, methods, ethics, management, timetable and resources. It emphasizes important considerations for each section like study design, population, analysis plan and limitations.