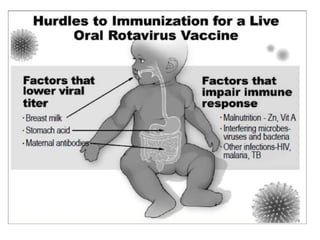
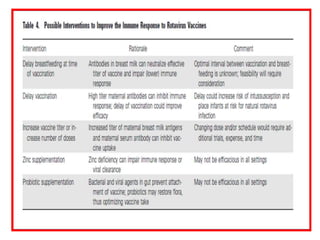
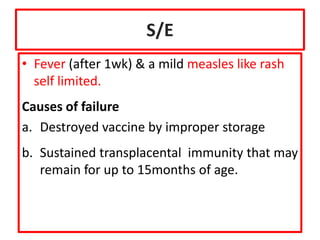
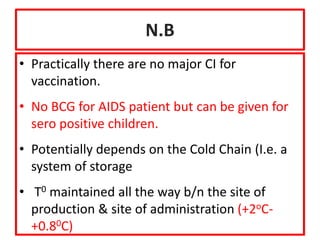
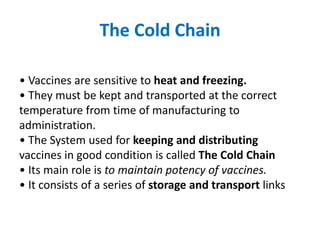
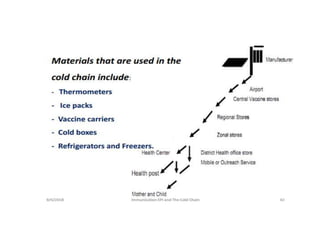
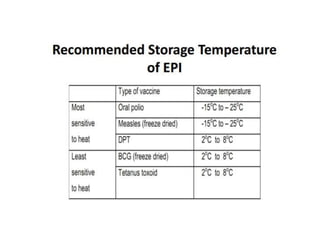
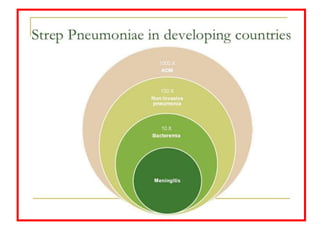
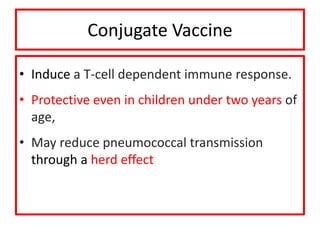
The document discusses various types of immunization including their goals, definitions, and details. It covers:
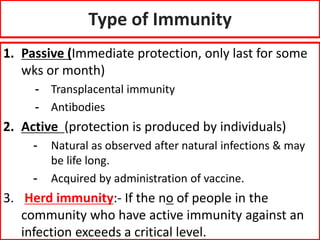
- Passive and active immunity from natural and artificial sources
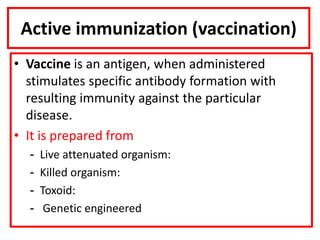
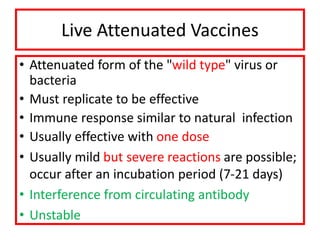
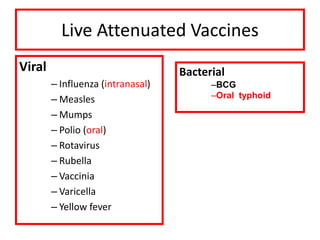
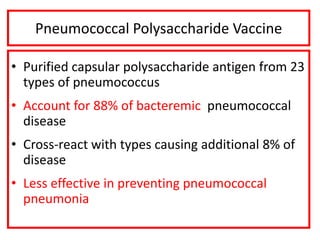
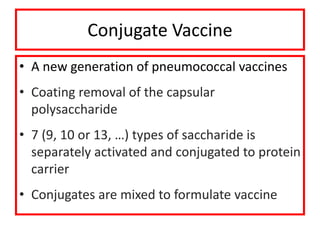
- Types of vaccines including live attenuated, inactivated, subunit, toxoid, and conjugate
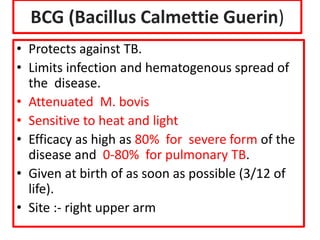
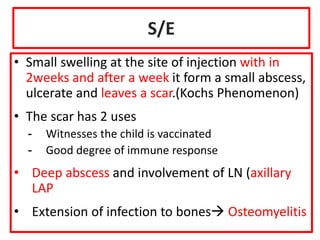
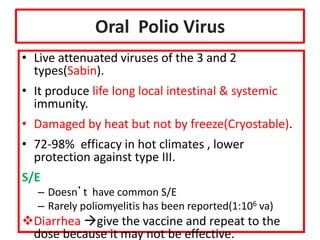
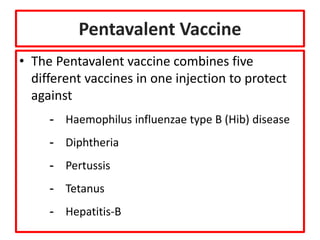
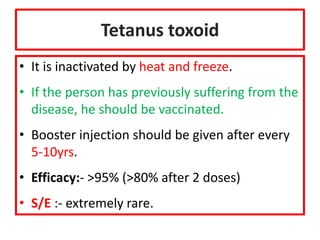
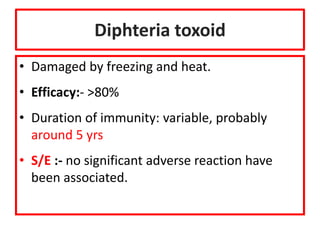
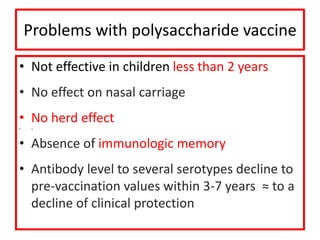
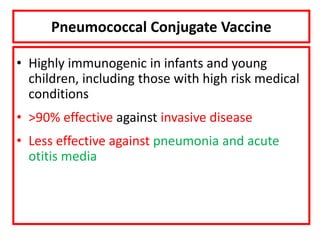
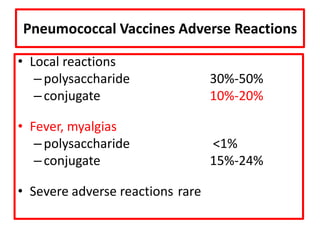
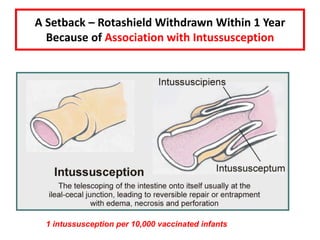
- Details on specific vaccines like BCG, polio, diphtheria, hepatitis B, pneumococcal, rotavirus, and measles
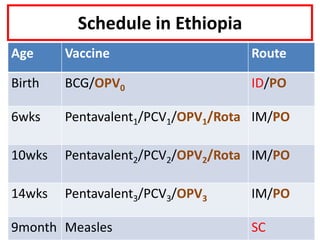
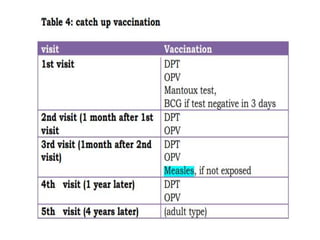
- Schedules, efficacy, advantages, side effects and contraindications of different vaccines
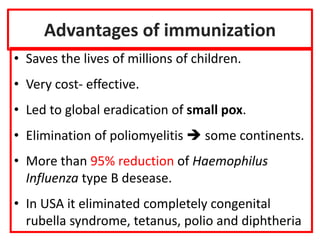
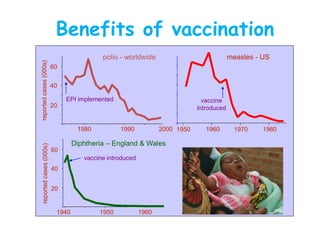
- The importance of vaccination programs in reducing disease prevalence globally



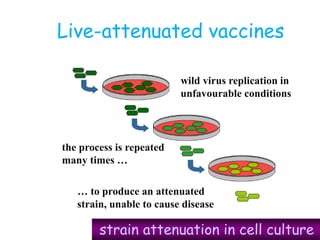
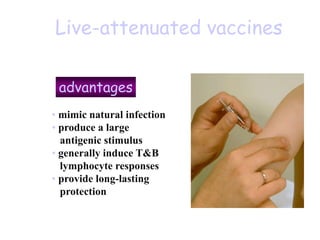

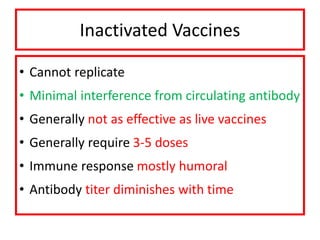
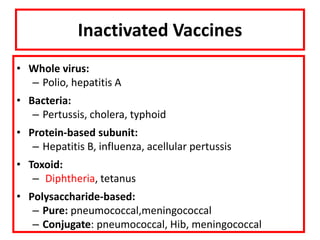
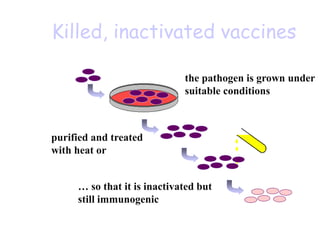

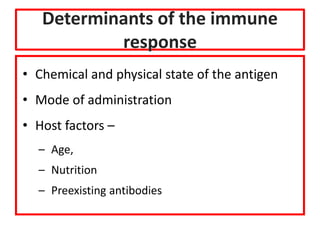





![Licensed Rotavirus Vaccines
Rotarix® RotaTeq®
Safety = =
Efficacy 90-100% severe
74-85% any severity
90-100% severe
74-85% any severity
Antigen Monovalent HRV
G1P[8]
Pentavalent
bovinehuman
reassortant
Schedule 2 doses 3 doses
Protection against
severe infx
2 years 2 years](https://image.slidesharecdn.com/1immunization-240209184647-b838bd90/85/1-Immunization-pptjjjjjjjhgjfxfffffddddd-44-320.jpg)