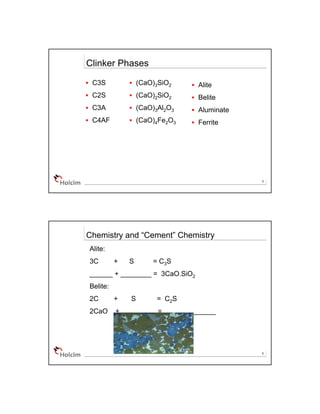
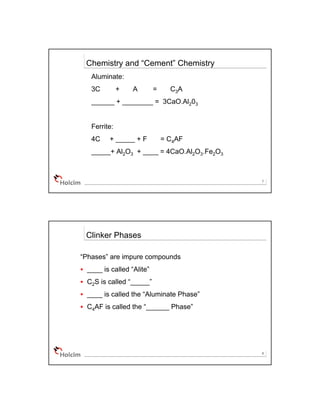
The document discusses cement chemistry and the key components and reactions involved. It introduces the common notation used - where elements like CaO are represented by single letters. It then explains the four main clinker phases produced by the reactions - C3S, C2S, C3A, C4AF. These correspond to the chemical compounds 3CaO·SiO2, 2CaO·SiO2, 3CaO·Al2O3, 4CaO·Al2O3·Fe2O3. It notes each phase's impact on the properties of cement such as strength, setting time, heat released.

![9
Impact of the phases
Clinker phase ‘Ideal’ content [%] Main effect on
C3S 60 - 65
Early strength
Final strength
C2S 15 - 5 Final strength
C3A 7 - 10
Setting and water demand
Heat of Hydration
C4AF 8 - 10 Color
Affects C3A (Iron Content)](https://image.slidesharecdn.com/1ecementchemistrynotation-190819181105/85/1-e-cement-chemistry-notation-5-320.jpg)