More Related Content
Similar to c10_1-3_particles-solids-liquids-gases.pdf
Similar to c10_1-3_particles-solids-liquids-gases.pdf (8)
More from seema chaturvedi
More from seema chaturvedi (8)
c10_1-3_particles-solids-liquids-gases.pdf
- 1. Copyright © TeacherNotes4U.com. All rights reserved. Do not allow to reproduce, distribute, display or publish the contents of this topic. Also not allow to alter or remove the contents in whole or part, in any form.
© TeacherNotes4U.com 版权所有。未经 TeacherNotes4U 事先书面同意,任何人不得复制、发行、传播、展示、出版或广播该等内容。亦不得更改或删除该内容复制品中所包含的商标、版权或其他声明。
© شر ن وال بع ط ال قوق حTeacherNotes4U.com. لك محفوظة الحقوق. الموضوع هذا محتويات نشر أو عرض أو توزيع أو إنتاج بإعادة تسمح ال
. شكل بأي ًايجزئ أو ًايكل المحتويات إزالة أو بتغيير ًاضأي تسمح ال
.
Year 10 Chemistry
1 / 2
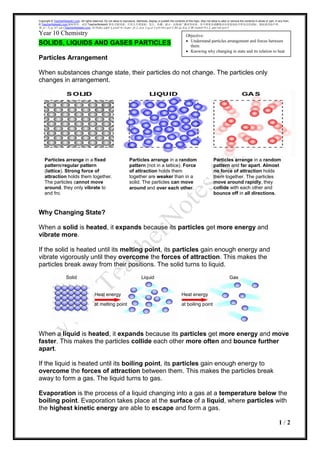
SOLIDS, LIQUIDS AND GASES PARTICLES
Particles Arrangement
When substances change state, their particles do not change. The particles only
changes in arrangement.
Why Changing State?
When a solid is heated, it expands because its particles get more energy and
vibrate more.
If the solid is heated until its melting point, its particles gain enough energy and
vibrate vigorously until they overcome the forces of attraction. This makes the
particles break away from their positions. The solid turns to liquid.
When a liquid is heated, it expands because its particles get more energy and move
faster. This makes the particles collide each other more often and bounce further
apart.
If the liquid is heated until its boiling point, its particles gain enough energy to
overcome the forces of attraction between them. This makes the particles break
away to form a gas. The liquid turns to gas.
Evaporation is the process of a liquid changing into a gas at a temperature below the
boiling point. Evaporation takes place at the surface of a liquid, where particles with
the highest kinetic energy are able to escape and form a gas.
Objective:
Understand particles arrangement and forces between
them
Knowing why changing in state and its relation to heat
Particles arrange in a fixed
pattern/regular pattern
(lattice). Strong force of
attraction holds them together.
The particles cannot move
around, they only vibrate to
and fro.
Particles arrange in a random
pattern (not in a lattice). Force
of attraction holds them
together are weaker than in a
solid. The particles can move
around and over each other.
Particles arrange in a random
pattern and far apart. Almost
no force of attraction holds
them together. The particles
move around rapidly, they
collide with each other and
bounce off in all directions.
Heat energy
at melting point
Heat energy
at boiling point
Solid Liquid Gas
- 2. Copyright © TeacherNotes4U.com. All rights reserved. Do not allow to reproduce, distribute, display or publish the contents of this topic. Also not allow to alter or remove the contents in whole or part, in any form.
© TeacherNotes4U.com 版权所有。未经 TeacherNotes4U 事先书面同意,任何人不得复制、发行、传播、展示、出版或广播该等内容。亦不得更改或删除该内容复制品中所包含的商标、版权或其他声明。
© شر ن وال بع ط ال قوق حTeacherNotes4U.com. لك محفوظة الحقوق. الموضوع هذا محتويات نشر أو عرض أو توزيع أو إنتاج بإعادة تسمح ال
. شكل بأي ًايجزئ أو ًايكل المحتويات إزالة أو بتغيير ًاضأي تسمح ال
.
Year 10 Chemistry
2 / 2
How Much Heat Energy?
The amount of heat energy needed for each substance to melt or boil is different.
This is because the particles for each substance are different, and with different
forces of attraction between them.
Increases in forces of attraction, increases the amount of heat energy needed to
overcome them. This increases the melting and boiling points.
forces of attraction heat energy melting point boiling point
Reverse Changing State
Above changing state by heating can be reversed by cooling.
When gas cools, it particles lose energy and move more slowly. The particles
collision becomes weaker to bounce away. So they stay close to form a liquid.
When liquid cools, it particles lose more energy and move even more slowly. At
freezing point, the slow moving particles will stop moving and vibrate on the spot only.
So they stay close and arrange in a regular pattern to form a solid. The strong forces of
attraction hold them tightly.