Mixtures
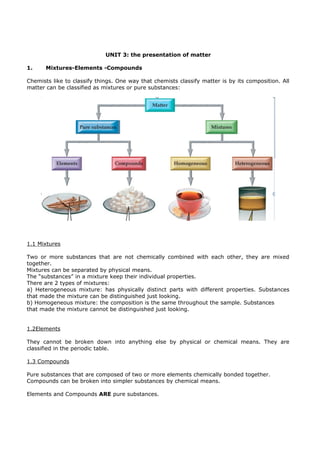
- 1. UNIT 3: the presentation of matter 1. Mixtures-Elements -Compounds Chemists like to classify things. One way that chemists classify matter is by its composition. All matter can be classified as mixtures or pure substances: 1.1 Mixtures Two or more substances that are not chemically combined with each other, they are mixed together. Mixtures can be separated by physical means. The “substances” in a mixture keep their individual properties. There are 2 types of mixtures: a) Heterogeneous mixture: has physically distinct parts with different properties. Substances that made the mixture can be distinguished just looking. b) Homogeneous mixture: the composition is the same throughout the sample. Substances that made the mixture cannot be distinguished just looking. 1.2Elements They cannot be broken down into anything else by physical or chemical means. They are classified in the periodic table. 1.3 Compounds Pure substances that are composed of two or more elements chemically bonded together. Compounds can be broken into simpler substances by chemical means. Elements and Compounds ARE pure substances.
- 2. 2. Separation Techniques •The separation of mixtures may be done to enhance the purity of substances •Accomplished using different characteristic properties, such as density, boiling point, melting point, solubility, etc 2.1 Filtration a) Technique used to separate mixtures of an insoluble solid and a liquid. b) Examples of mixtures you can separate using filtration: sand and water, broken glass and water 2.2 Crystallization Is the process in which crystals are formed. Crystallization is based on the principles of solubility: compounds (solutes) tend to be more soluble in hot liquids (solvents) than they are in cold liquids. If a saturated hot solution is allowed to cool, the solute is no longer soluble in the solvent and forms crystals of pure compound. Impurities are excluded from the growing crystals and the pure solid crystals can be separated from the dissolved impurities by filtration. 2.3 Magnetic separation
- 3. Is a process in which magnetically susceptible material is extracted from a mixture using a magnetic force. This separation technique can be useful in mining iron as it is attracted to a magnet. 2.4 Distillation This is good for separating two or more liquids from each other. For example, ethanol (alcohol) can be separated from a mixture of ethanol and water by distillation. This method works because the two liquids have different boiling points. To separate a mixture of liquids, the mixture can be heated to force one component, which have different boiling points, into the gas phase. The gas is then condensed back into liquid form and collected. 2.5 Decantation A mixture of two immiscible liquids can be separated using a separating funnel, the working of which is based on the differences in the densities of the liquids. The heavier liquid which settles below is drained out first from below the funnel into a beaker, and then the lighter liquid is drained out into another beaker.
- 4. 3. Solutions (Homogeneous mixtures) A simple solution is basically two substances that are evenly mixed together. One of them is called the solute and the other is the solvent. A solute is the substance to be dissolved (sugar). The solvent is the one doing the dissolving (water). As a rule of thumb, there is usually more solvent than solute. Be patient with the next sentence as we put it all together. The amount of solute that can be dissolved by the solvent is defined as solubility. That's a lot of "sol" words. 3.1 Expressing the concentrations of solutions a) Mass Percent The mass percent is used to express the concentration of a solution. Indicates how many grams of solute are in 100 grams of solution. Formula: Mass of solute Mass Percent= x 100 Mass of Solution b) Volume percent The volume percent is used to express the concentration of a solution. Indicates the solute volume that is in 100 volume units of solution. Volume of solute Volume Percent= x 100 Volume of Solution
- 5. c) Mass concentration Indicates the mass of solute that is in every volume unite of solution. Formula: Mass of solute Mass concentration= Solution volume 4. The atomic molecular theory of Dalton Early humans easily distinguished between materials that were used for making clothes, those that could be shaped into tools, or those that were good to eat. Then they gave these things the names, such as "fur," "stone," or "rabbit." However, these people did not have our current understanding of the substances that made up those objects. Democritus, a Greek who lived from 460 BCE to 370 BCE, developed a new theory of matter that attempted to overcome the problems of his predecessors. Democritus's ideas were based on reasoning rather than science, and drew on the teachings of two Greek philosophers who came before him: Leucippus and Anaxagoras. Democritus knew that if you took a stone and cut it in half, each half had the same properties as the original stone. He reasoned that if you continued to cut the stone into smaller and smaller pieces, at some point you would reach a piece so tiny that it could no longer be divided. Democritus called these infinitesimally small pieces of matter atoms, meaning 'indivisible'. He suggested that atoms were eternal and could not be destroyed. Democritus theorized that atoms were specific to the material that they made up, meaning that the atoms of stone were unique to stone and different from the atoms of other materials, such as fur. Later, John Dalton, an exceptional British teacher and scientist, put together the pieces and developed the first modern atomic theory in 1803. Dalton realized that water could exist as a gas that mixed with air and occupied the same space as air. Solids could not occupy the same space as each other; for example, ice could not mix with air. So what could allow water to sometimes behave as a solid and sometimes as a gas? Dalton realized that all matter must be composed of tiny particles. In the gas state, those particles floated freely around and could mix with other gases. But Dalton extended this idea to apply to all matter – gases, solids and liquids. Dalton first proposed part of his atomic theory in 1803 and later refined these concepts in his classic 1808 paper A New System of Chemical Philosophy.
- 6. Dalton's Elements 4.1 Dalton's theory had four main concepts 1. All matter is composed of indivisible particles called atoms: Dalton pictured atoms as tiny billiard-ball-like particles in various states of motion. While this concept is useful to help us understand atoms, it is not correct as we will see in later modules on atomic theory linked to at the bottom of this module. 2. All atoms of a given element are identical; atoms of different elements have different properties. Dalton's theory suggested that every single atom of an element such as oxygen is identical to every other oxygen atom; furthermore, atoms of different elements, such as oxygen and mercury, are different from each other. 3. Chemical reactions involve the combination of atoms, not the destruction of atoms. Atoms are indestructible and unchangeable, so compounds, such as water and mercury calyx, are formed when one atom chemically combines with other atoms. This was an extremely advanced concept for its time; while Dalton's theory implied that atoms bonded together, it would be more than 100 years before scientists began to explain the concept of chemical bonding. 4. Compounds: Most of the materials we come into contact with are compounds, substances formed by the chemical combination of two or more atoms of the elements. A single "particle" of a compound is called a molecule. Dalton incorrectly imagined that atoms "hooked" together to form molecules. However, Dalton correctly realized that compounds have precise formulas. Water, for example, is always made up of two parts hydrogen and one part oxygen. The chemical formula of a compound is written by listing the symbols of the elements together, without any spaces between them. If a molecule contains more than one atom of an element, a number is subscripted after the symbol to show the number of atoms of that element in the molecule. Thus the formula for water is H2O, never HO or H2O2.
- 7. Dalton's Elements 4.1 Dalton's theory had four main concepts 1. All matter is composed of indivisible particles called atoms: Dalton pictured atoms as tiny billiard-ball-like particles in various states of motion. While this concept is useful to help us understand atoms, it is not correct as we will see in later modules on atomic theory linked to at the bottom of this module. 2. All atoms of a given element are identical; atoms of different elements have different properties. Dalton's theory suggested that every single atom of an element such as oxygen is identical to every other oxygen atom; furthermore, atoms of different elements, such as oxygen and mercury, are different from each other. 3. Chemical reactions involve the combination of atoms, not the destruction of atoms. Atoms are indestructible and unchangeable, so compounds, such as water and mercury calyx, are formed when one atom chemically combines with other atoms. This was an extremely advanced concept for its time; while Dalton's theory implied that atoms bonded together, it would be more than 100 years before scientists began to explain the concept of chemical bonding. 4. Compounds: Most of the materials we come into contact with are compounds, substances formed by the chemical combination of two or more atoms of the elements. A single "particle" of a compound is called a molecule. Dalton incorrectly imagined that atoms "hooked" together to form molecules. However, Dalton correctly realized that compounds have precise formulas. Water, for example, is always made up of two parts hydrogen and one part oxygen. The chemical formula of a compound is written by listing the symbols of the elements together, without any spaces between them. If a molecule contains more than one atom of an element, a number is subscripted after the symbol to show the number of atoms of that element in the molecule. Thus the formula for water is H2O, never HO or H2O2.