This whitepaper discusses the importance of interaction design in medical systems, emphasizing user-centered design to minimize use-related errors and enhance user satisfaction. It outlines essential methods, such as user research and iterative design, while providing regulatory perspectives that support design activities. The document stresses that understanding user needs is critical for successful medical product development and highlights the role of design ethnography in identifying user motivations and workflows.


![1. Introduction
DOUBLE-EDGED SWORD OF CHANGE
When products are hard to use, they cause people
pain. That pain may be largely psychological, as in the
frustration of a nurse who has to enter a standard
value into a system over and over. But in the case of
some critical medical systems, that pain may actually
be physical: picture the despair of an elderly patient
who lives alone when she fails to tighten a small
connector with her arthritic fingers.
Have you ever sat in product development meetings where several
team members expressed strong and differing opinions about
what “the user” really wants? Have you ever experienced delays
while team members struggle to articulate and finalize product
requirements? Or have you ever managed a project where the basic
product mandate changed late in the development phase?
Perhaps you feel the pain of your customers’ bitter
complaints, or the squeeze of disappointing sales
figures. Perhaps you recognize that the medical
systems you develop aren’t easy to use. Such painful
insights are the first steps towards embracing change.
As somebody in charge of planning and managing medical system
development, you’re solving challenging problems on a daily basis.
You have to balance efficacy with safety, and build products your
customers actually want to buy. You also need to ensure regulatory
compliance while controlling development costs and getting
your product to market as fast as possible. Adding complexity to
today’s medical systems, they often involve a large measure of
user interactivity, whether your users are physicians, clinicians,
administrators or patients. The last things you need are unclear
requirements and shifting mandates.
Change isn’t always easy. It’s rare that people change
simply for the good of changing, and research has
shown that people are usually only ready for change
when they actively feel pain in their current situation.
[Nadler]
Help is at hand from the discipline known as Interaction Design.
Interaction Design practitioners work to ensure that your users will
have a satisfying experience with your medical products and services.
Interaction Design offers tools that help you determine what your
users really need and applies processes that deliver solid solutions.
Introducing Interaction Design activities to your
medical system development process likely means
bringing change to your organization. It isn’t always
going to be easy, but it’s far preferable to pursue
this effort now, before some extremely painful event
— such as failing to obtain pre-market approval or
suffering a product recall — forces it upon you.
This whitepaper first will explain that regulatory agencies regard
design activities for medical systems in a very positive light. Then
we’ll discuss the innovative nature of design thinking by comparing
Interaction Design to more well-known engineering disciplines.
Next, several key Interaction Design methods that benefit product
development will be introduced, supported by case study examples.
These methods include: user research; the iterative design process;
and requirements documentation. Finally, this whitepaper considers
some ways that Interaction Design practitioners can operate
successfully within a large organization.
Whitepaper • Interaction Design for Medical Systems
3](https://image.slidesharecdn.com/2011-02-242ixdformedicalsystems-110224151029-phpapp02/85/Interaction-Design-for-Medical-Systems-Whitepaper-3-320.jpg)
![“Simply put, the risk of having use-related errors in
medical systems is minimized when the organization
understands its users and fully incorporates design
processes into the product development lifecycle.”
2. Regulatory Perspective
on
Design
The American and European regulatory agencies FDA and TÜV both
espouse a user-centered approach to medical system development
that is strongly in keeping with the core principles and practices of
Interaction Design. The FDA writes that: “The majority of use error is
human in origin, and these mistakes can often be attributed to poor
system design.” [FDA] Refer also to the figure at left for the IEC’s
depiction of how human-centered design controls should operate to
inform an iterative development cycle. Happily, this iterative process
is a fundamental tenet of Interaction Design practice.
One of the most pertinent American regulatory documents
considered mandatory today is entitled “Medical Device Use-Safety:
Incorporating Human Factors Engineering into Risk Management:
Identifying, Understanding and Addressing Use-Related Hazards.”
[Kaye] Regulatory professionals have likely read the other most
relevant publications [CDRH, FDA, Sawyer]. The international IEC
60601-1 standard on Risk Management and collateral standard 606011-6 on Usability as well as AAMI HE-75:2009, a companion standard to
HE-74:2001, Human factors design process for medical devices. also
point to the positive results that design processes offer by focusing
on users throughout iterative development stages and thus mitigating
risk.
Regulatory model of good human-centered design process [IEC]
The regulatory agencies’ focus on the risk of use-related hazards is
noteworthy. Calculating the return on investment (ROI) for design
activities is notoriously difficult due to the challenge of isolating
the impact of design activities among other product development
activities. Many experts have concluded that ROI is the wrong
metric by which to judge design because it’s a tactical measurement
whereas design is a strategic initiative. [Dray] Instead, we can
reframe the rationale for design as one of risk management. Simply
put, the risk of having use-related errors in medical systems is
minimized when the organization understands its users and fully
incorporates design processes into the product development lifecycle.
Whitepaper • Interaction Design for Medical Systems
4](https://image.slidesharecdn.com/2011-02-242ixdformedicalsystems-110224151029-phpapp02/85/Interaction-Design-for-Medical-Systems-Whitepaper-4-320.jpg)
![3. From Engineering
to
Design
The field of Interaction Design seeks to improve the human condition
by improving people’s interactions with technology. Interaction
Design focuses on defining the behaviors of interactive products and
systems based on an understanding of how and why people need to
use them. In defining system behaviors, Interaction Design addresses
the system’s presentation to users and how that presentation makes
people feel and respond. The essential practices of Interaction Design
address the hands-on user experience.
The first use of the term “Interaction Design” in the mid1980s is credited to Bill Moggridge, founder of IDEO, along
with pioneering designer Bill Verplank. [Moggridge 14] Alan
Cooper advanced and popularized the discipline by founding
a dedicated consultancy called Cooper Interaction Design in
1992 and publishing two seminal books on the subject. [Cooper
et al.; Cooper]
In the medical industry, the disciplines of Human Factors
Engineering and Usability Engineering are much more
frequently represented in the organization compared to
Interaction Design. Human Factors Engineering originated
during the 1940s in the aeronautical industry, along with
the study of ergonomics. It aims to ensure that systems
accommodate the cognitive and perceptual limitations of
human beings. Usability Engineering evolved later within the
academically-grounded field of study called Human-Computer
Interaction. Its core methods focus on analyzing, testing and
inspecting systems for maximal usability.
The daunting complexity of World War II cockpits such as this Boeing
B-17G Flying Fortress caused fatal crashes, which showed the
importance of having machines adapt to human constraints instead of
forcing people to adapt themselves to machines.
Although both Human Factors and Usability Engineering reach
out towards the design of interactive systems, these engineeringbased disciplines emphasize analytical and evaluative skills versus
creative and generative skills. Instead of trying to measure users’
abilities and test system performance, Interaction Design seeks to
understand users’ needs and motivations in order to inform system
Whitepaper • Interaction Design for Medical Systems
5](https://image.slidesharecdn.com/2011-02-242ixdformedicalsystems-110224151029-phpapp02/85/Interaction-Design-for-Medical-Systems-Whitepaper-5-320.jpg)
![behavior. Interaction Design delivers tangible user-centered insights
and product innovation using methods that blend analytical and
creative thinking.
Just as the study of medicine and science is undergoing a shift from
a Newtonian, mechanical-world orientation to a more Darwinian,
biological-world orientation, product development today is
experiencing a shift from engineering-based practices to designbased practices. The power of design thinking is reaching critical
mass with technology business leaders today as a key differentiator
in competitive marketplaces. [Wasserman] Although some novel
medical technologies may not be faced with a crowded market,
that’s likely to be a temporary state of affairs. Today, we can observe
rapid commodification of even such sophisticated medical systems as
insulin pumps or pacemakers.
“The essential practices of Interaction Design address
the hands-on user experience. Satisfied users make for
successful companies. The medical industry is ripe for a
strong company to stake a claim to great design.”
COMPANY GAIN IN VALUE
(2000-2004)
Most people desire to have a good-quality experience with the
systems they use. Popular online services such as Get Satisfaction
and Angie’s List let people educate themselves about the quality of
products and services before making their purchase decision. The
rise of social networking services also magnifies the speed with which
bad user experiences can be broadcast to the world. Medical systems
are not going to be immune from this broader movement. On the
contrary, the medical industry appears ripe for a strong company to
stake a claim to great design, the way Apple has claimed that ground
in computing technology and consumer devices.
Satisfied users make for successful companies. Recent studies by
researchers at the University of Michigan showed that a portfolio of
companies with the happiest customers gained 75% in value from
2000 to 2004, dwarfing the 19% gain for the Standard & Poor’s 500
Index during the same period; happy-customer companies were also
less volatile and experienced less employee turnover. [MSN]
Happy customers deliver corporate value [MSN]
Whitepaper • Interaction Design for Medical Systems
6](https://image.slidesharecdn.com/2011-02-242ixdformedicalsystems-110224151029-phpapp02/85/Interaction-Design-for-Medical-Systems-Whitepaper-6-320.jpg)
![“User research involves understanding the behaviors,
characteristics, motivations and needs of the
envisioned system’s end users. It doesn’t take a long
time or cost a lot of money.”
Great medical products and services are viable,
feasible — and also satisfying to use
4. Power
of
User Research
Research is central to user-centered design thinking and practice.
While marketing departments often conduct research to assess the
viability of selling a product and engineering departments often
conduct research to assess the feasibility of building a product, it
often happens that nobody is responsible for conducting research to
understand what makes a product satisfying to use. Knowing how
to satisfy users is different than measuring a product’s usability.
Usability is a very important quality of interactive systems, but it is
typically assessed part-way through product development or even
later when a working system is available. By contrast, user research
should be conducted prior to finalizing your product’s scope and
certainly prior to defining the user experience.
User research may not yet be widely applied in medical system
development but it is a powerful strategic force for innovation.
Unlike most marketing or academic research, user research
is qualitative in nature. Such research involves obtaining an
understanding of the behaviors, characteristics, motivations and
needs of the envisioned system’s end users. Qualitative research
methods differ markedly from quantitative ones such as surveys or
clinical trials because statistical validity is neither expected nor
sought. For medical industry professionals, qualitative research may
seem disconcertingly “fuzzy,” but obtaining useful customer data
from small sample sizes has a solid history of successful application to
product design. [Beyer et al. 38; Calde]
Interaction Designers are trained to identify the behaviors of people
and characteristics of the system that matter most to product
success. Whether your current development effort involves groundbreaking trials to validate a novel algorithm or releasing iteration
seven of a well-established product, user research with your target
customers will reveal what people will find satisfying to use. It
doesn’t take a long time or cost a lot of money. Clarifying the scope
of your problem space up-front through user research methods can
be extremely helpful in order to focus the later efforts of more costly
implementation and production resources.
Whitepaper • Interaction Design for Medical Systems
7](https://image.slidesharecdn.com/2011-02-242ixdformedicalsystems-110224151029-phpapp02/85/Interaction-Design-for-Medical-Systems-Whitepaper-7-320.jpg)
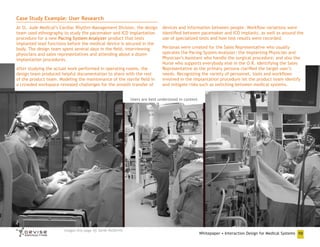
![Research Method: Ethnography
Less Appropriate Research Tools:
Focus Groups & Surveys
Two research methods that can be inappropriately
applied to front-end product definition are focus
groups and surveys.
Focus groups are a qualitative research tool, involving
a moderator presenting questions and inputs to a
group of people in a controlled environment. Focus
groups don’t provide good design input because
designers need to learn about the actual, specific
behaviors of individuals, not the quick responses
of artificially-gathered groups. People are also
notoriously bad at self-reporting behaviors, and what
users say may differ significantly from what they
do. [Nielsen] Lastly, focus groups can tend to be
dominated by one or two strong-voiced individuals.
Generally, focus groups should be reserved for
addressing early-stage marketing issues such as
product viability and branding.
Surveys are a quantitative tool where a hopefullylarge set of people respond to a set of questions
posed by a researcher. They suffer from a basic
limitation in that they collect simple answers only
to the questions asked, so they will tend to garner
people’s opinions about surface issues. Again,
they don’t provide deep insights about people’s
behaviors and goals needed to properly define
interactive systems. Generally, surveys should be
reserved for addressing product management issues
such as competitive benchmarking or feature list
prioritization.
Ethnography is a powerful qualitative research method that delivers
deep, valuable information about your user population in a relatively
short period of time. As a user research tool, ethnography leverages
techniques developed in the fields of anthropological and sociological
research. Its practice involves designers & other product stakeholders
visiting users’ environments to observe and interview individuals
performing the activities in question.
Personal engagement between the designer and user helps to uncover
the motivations that drive people to perform and the specific design
qualities that will result in satisfied customers. Design ethnography
conducted before your medical system development effort begins
helps to reveal product opportunities. Such direct observation of
users is an essential component of the applied design thinking that
generates innovative solutions. [Wasserman]
One of the key factors that design ethnography uncovers relates to
people’s contexts of use. Contexts of use are those environments
where users will interact with your product or service. Understanding
the medical context of use especially helps to identify risk areas that
must be mitigated. Medical systems are frequently used in special
institutional settings such as hospitals and clinics that often involve
specific functional constraints such as 3rd-party systems being used in
conjunction with yours. Conducting first-hand research in the field is
essential to identifying such environmental issues.
The artifacts and models produced by Interaction Designers as
outputs from user research are retained during all subsequent
product development efforts. Such data-driven models establish a
user-centered foundation that helps keep teams on-target with their
feature-level decisions during later stages of product development.
Those often-frustrating opinions from various team members about
what “the user” wants can be assessed in light of real-world data
collected in the field. Documenting user research allows your entire
organization to benefit from the work of the research team.
Whitepaper • Interaction Design for Medical Systems
8](https://image.slidesharecdn.com/2011-02-242ixdformedicalsystems-110224151029-phpapp02/85/Interaction-Design-for-Medical-Systems-Whitepaper-8-320.jpg)
![Research Model: Personas
EXAMPLE PERSONA
One of the most powerful user research models that can be built
from design ethnography is called a Persona. Personas are fictional
user archetypes. Persona descriptions may read like a story but they
are properly based on research data. [Bacon et al.] Personas embody
essential behavioral patterns among target users. Using a friendly
and approachable narrative format, they describe users’ key tasks,
attitudes, motivations and workflows.
Personas help identify product opportunities and clarify use-related
risks. The activities of design ethnography involve understanding
people’s motivations and existing workflows. Personas embody those
findings. Analyzing why people do things and how people do things
can generate innovative solutions for ways that the newly-designed
system can support personas’ goals more directly. Personas reveal
when the new product can reduce or eliminate any frustrating or
redundant steps in the user’s current workflow.
Rich, data-driven personas also facilitate communication and usercentered decision-making. They allow the product team to share
a common understanding of target users. Team members can now
reference a specific individual persona and ground their decisions in
a shared understanding of who exactly is being served. There are no
more arguments over what “the user” wants, because specific users
are clearly understood by the whole team. Because realistic personas
generate empathy, they also encourage team members to remain
committed to delivering a high-quality, satisfying user experience.
Personas are also a powerful planning tool. Personas help product
managers prioritize target users and control scope. The designated
primary persona has needs that must be served entirely by the design
solution, while secondary personas have additional needs that must
be served in some area. The needs of supplemental personas, who
are fully served by the solutions devised for primary and secondary
personas, are not allowed to impact the design solutions. Personas
also work seamlessly with a scenario-based approach to Interaction
Design, which will be discussed in the following chapter.
Dr. Helena Reardon
Interventional Cardiologist
Dr. Reardon performs a variety of surgical procedures
every day starting at 7am. She sees her daily schedule
when she arrives to the hospital and scrubs down
outside the operating room. Most procedures
are relatively routine surgical ablations and device
implantations, but her case load of biventricular
implants has been increasing. Biventricular implants
can take hours to complete since placement of
the left ventricular lead involves tricky intravenous
navigation. She welcomed the introduction of RF
telemetry to remove the barrier of the sterile field
between herself and industry representatives, but
she’s frustrated whenever telemetry is lost because it
slows her down. Dr. Reardon’s goals include:
•
•
•
Have information at her fingertips
Trust her tools
Experience no surprises
This abbreviated persona description was developed for example
purposes only and is property of Devise LLC
Whitepaper • Interaction Design for Medical Systems
9](https://image.slidesharecdn.com/2011-02-242ixdformedicalsystems-110224151029-phpapp02/85/Interaction-Design-for-Medical-Systems-Whitepaper-9-320.jpg)



![5.2. DESIGN FRAMEWORK PHASE
PRINCIPLES AND PATTERNS
Interaction Design practitioners will refer
to proven principles and patterns during the
Modeling stage. Noteworthy principles and
patterns include:
• Don’t make the user feel stupid. [Cooper 97]
This principle may capture the moral essence
of interaction design and is extraordinarily
pertinent for medical systems used by
physicians.
• Optimize for intermediates. [Cooper 45]
Few users remain beginners and few users
become experts — the majority of users exist
somewhere in-between, and need be served
with interaction mechanisms suited to their
level of expertise.
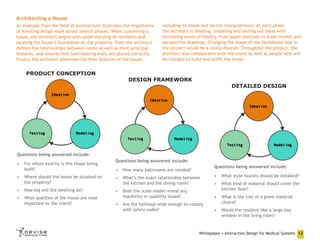
After the foundation is laid for the product concept, the Design
Framework phase involves defining the overall user experience.
Design Framework Modeling produces manifestations of the product
features that are deemed in-scope, while Testing at this juncture
validates design choices and identifies areas for further refinement.
Design Framework: Ideation
Interaction Design practitioners can use the scaffolding of usage
scenarios and persona goals to conceive the appropriate design
framework. The ultimate user experience must deliver the right
functionality in the right form at the right time. Ideation at this
phase tends to be approached both visually and verbally, using a
combination of analytical and creative tools to address how the
system can best serve users needs. If this description sounds abstract,
that’s because ideation here is a relatively intangible design stage —
the artifacts that you can see are delivered through Modeling, next.
Design Framework: Modeling
• Global navigation. [Tidwell 66] Devote an
appropriate portion of each interface to a set
of controls that take the user to key sections
of the application. This pattern gives control
to users and provides reassuring consistency.
Scenarios are expanded and deepened through successive conceptual
iterations. High-level usage scenarios are broken down into key path
scenarios. On the visual side, initial whiteboard or pencil sketches
become digital wireframes. Wireframes are digital representations of
rough product functionality, with a low level of visual fidelity. Usually
they will consist of black-and-white outlines of key system states.
• Progress indicators. [Tidwell 149] Show the
user how much progress is being made for any
operation lasting longer than 2 seconds, even
if the time estimate is imprecise. This pattern
relieves user anxiety and makes people more
patient.
Many designers will also proceed on to static mockups at this
juncture. Mockups are higher-fidelity representations of the
system, which integrate more visual polish into the product design.
Storyboards can also bring scenarios and personas to life when a
designer is handy with drawing figures or has access to life-size
models.
One of the more important tools commonly employed by Interaction
Design practitioners modeling the design framework include
principles and patterns. These may come from experts and
publications, while others are developed as internal design standards
Whitepaper • Interaction Design for Medical Systems 16](https://image.slidesharecdn.com/2011-02-242ixdformedicalsystems-110224151029-phpapp02/85/Interaction-Design-for-Medical-Systems-Whitepaper-16-320.jpg)
![for product families or exist as competitive standards within
established industries. Some medical design frameworks, such as
games that are intended to promote health or products aimed at
engendering a healthy lifestyle, may also need to consider emotional
affects such as playfulness and fun to ensure user engagement and
patient compliance.
DESIGN FRAMEWORK PHASE
Design Framework: Testing
At this phase, Testing generally involves additional user research. As
the product models gain fidelity, a qualitative user research method
called Usability Testing can be applied to refine and validate proposed
solutions. Usability testing is a way of measuring usability and user
satisfaction by presenting potential and current users with some form
of product model. These product models vary in resolution depending
on the maturity of the project; at the Design Framework phase they
will tend to comprise paper prototypes of the wireframes or static
mockups but could involve an interactive prototype.
Usability Testing involves presenting product models to users in
experimentally-controlled, one-on-one sessions. Such testing with
real people can reveal a wealth of information about the design
framework’s strengths and weaknesses. Usability Testing is a proven
rapid and cost-effective research technique. Respected studies of
usability testing show that sessions conducted with as few as five
users can reveal 85% of a product’s total usability problems. [Nielsen]
Applied in an iterative fashion, multiple rounds of usability testing
during the Design Framework phase can augment the design process.
To summarize the results for team consumption, usability test reports
will highlight users’ response patterns and identify areas of the
product that could use further design refinement.
Design process methods and outputs for Design Framework phase
“The Design Framework phase involves defining the overall
user experience. Designers use wireframes and mockups
and apply principles and patterns to model solutions.
Usability Testing is a proven rapid and cost-effective
research technique to validate the design framework.”
Whitepaper • Interaction Design for Medical Systems 17](https://image.slidesharecdn.com/2011-02-242ixdformedicalsystems-110224151029-phpapp02/85/Interaction-Design-for-Medical-Systems-Whitepaper-17-320.jpg)





![7. Organizational Structure
Medical systems development is anything but a solo endeavor,
and products frequently take multiple years to move from being
a concept in someone’s mind to being released in the market. To
deliver the most value, the Interaction Design practitioner should be
a core team member from the first moments of problem identification
through the end game of market launch. Generally, the discipline of
Design (which may include Interaction Design; Industrial Design and/
or Visual Design) should have department-level representation in the
organization.
Establishing a solid foundation of user research about people’s needs
enables teams to make better decisions without requiring constant
direct user input, as discussed above. Throughout the development
process, the Interaction Design practitioner becomes principally
charged with representing the user’s perspective. However, users’
needs are not the only decision-driver in product development.
Interaction Design practitioners must know how to balance the
concerns of business and technology with those of users. Listening
and collaboration are key Interaction Design skills for forging a shared
team vision. Most practitioners are well-versed in cross-disciplinary
teamwork. The importance of successful collaboration is established
in academic design programs as well as being a best practice in
industry. [Corporate]
In any serious medical systems company, a centralized
Interaction Design team will collaborate with many parts of the
organization (other departments’ collaborations not depicted)
Interaction Design practitioners tend to operate at the intersection of
various departments, as shown in the diagram at left. This situation
is especially true for medical systems development because the
initial vision for products usually begins within clinical, research or
marketing departments while the end game of implementation is
usually the responsibility of engineering departments.
Interaction Design is not properly a marketing function, however,
since marketing is focused on product viability and selling, nor is
it properly an engineering function, which is focused on product
feasibility and implementation.
Whitepaper • Interaction Design for Medical Systems 23](https://image.slidesharecdn.com/2011-02-242ixdformedicalsystems-110224151029-phpapp02/85/Interaction-Design-for-Medical-Systems-Whitepaper-23-320.jpg)



