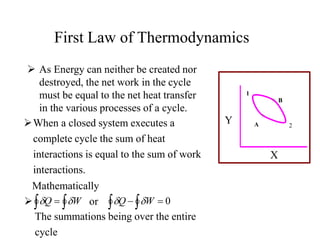
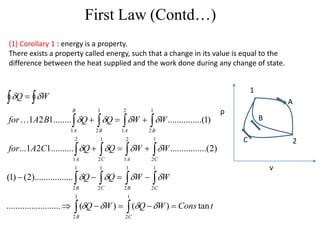
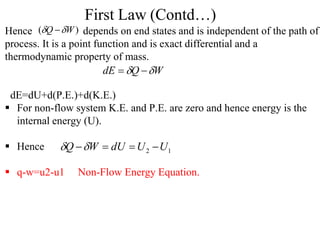
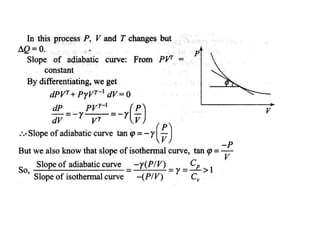
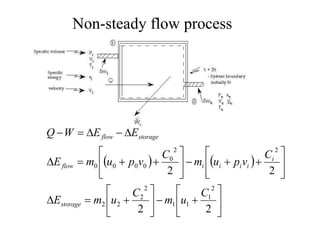
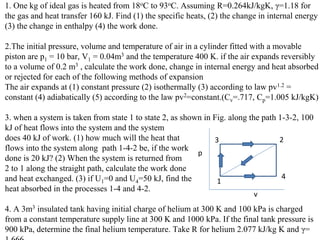
The document discusses the first law of thermodynamics. It states that the net work done by a system in a cycle must equal the net heat transfer into the system for the cycle. Mathematically, this can be written as the sum of all work interactions equals the sum of all heat interactions for the entire cycle. The first law also establishes that energy is conserved and can be changed from one form to another but cannot be created or destroyed. It discusses various thermodynamic processes and applications of the first law including steady flow processes.