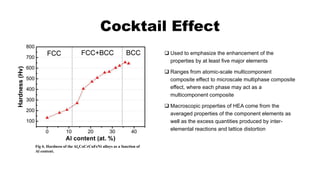
The document provides an in-depth overview of high entropy alloys (HEAs), detailing their composition, core effects, mechanical properties, processing routes, and applications. It describes the unique characteristics of HEAs, such as sluggish diffusion, lattice distortion, and the cocktail effect, which contribute to their enhanced mechanical properties. Applications of HEAs include coatings, bulk metallic glasses, and carbides, highlighting their strength, corrosion resistance, and thermal stability.
![High Entropy Alloys
Chayon Mondal
Roll no.: 16142006
M.Tech – I [Alloy Technology]
Metallurgical Engineering
Indian Institute of Technology (Banaras Hindu University), Varanasi](https://image.slidesharecdn.com/highentropyalloys-170424111227/75/High-entropy-alloys-1-2048.jpg)